Norwegian translation of a new, patient-reported multidimensional questionnaire on dyspnoea
Summary
Background: Self-reported dyspnoea screening is important for following up and treating a number of conditions, but there was no validated Norwegian instrument for measuring multiple dimensions of dyspnoea.
Objective: To translate and linguistically validate the Multidimensional Dyspnea Profile (MDP).
Method: The Norwegian translation and linguistic validation were carried out in accordance with international guidelines in collaboration with the MDP copyright holder and Mapi, which specialises in the translation and linguistic validation of patient-reported end points. The Norwegian translation was performed by two lung specialists: a pulmonary nurse and an intensive care nurse. The back translation into English was carried out by a professional translator and a bilingual native English speaker. We conducted structured interviews with five patients with dyspnoea in order to check that the instrument was clearly worded and easy to understand.
Results: The Norwegian version of the MDP includes 11 measurable themes: immediate discomfort from breathing sensations, presence and intensity of five different types of dyspnoea and intensity of five types of emotional responses to dyspnoea. The developers of the MDP own the copyright, but the instrument is open access for non-commercial use via Mapi’s website.
Conclusion: The MDP is the first instrument in Norwegian for measuring multiple dimensions of dyspnoea, regardless of underlying medical conditions. The MDP should be validated using a larger patient sample with a variation in diagnoses, age and medical conditions. The instrument can be useful in research and clinical interventions.
Cite the article
Haugdahl H, Knutli M, Sorger H. Norwegian translation of a new, patient-reported multidimensional questionnaire on dyspnoea. Sykepleien Forskning. 2020;15(81376):e-81376. DOI: 10.4220/Sykepleienf.2020.81376en
Dyspnoea is defined as a subjective experience of not being able to breathe properly that entails qualitatively different emotions and sensations of varying intensity. Satisfactory screening for dyspnoea is dependent on self-reporting (1). Dyspnoea affects up to 50 per cent of emergency department patients, and a quarter of patients considered to be ambulant (1).
In a study by St. Olav’s Hospital, dyspnoea was the third most common symptom (9 per cent) among patients referred to the emergency department, after abdominal pain (13 per cent) and chest pain (13 per cent) (2).
Important predictor of morbidity and mortality
Dyspnoea is an important predictor of morbidity and mortality (3–6), including cause-specific death from heart or lung disease (7).
A prospective multicentre study showed that self-reported dyspnoea is a better predictor of five-year survival in patients with chronic obstructive pulmonary disease (COPD) than physiological measurements of forced expiratory volume after one second (FEV1) (4).
Differing experiences of dyspnoea
Dyspnoea manifests itself in different forms, depending on which stimuli are causing breathlessness and which neurophysiological networks are sending stimuli back to the brain (8). People with the same medical condition can also have very different experiences of dyspnoea (8, 9).
Impaired lung function measured using FEV1 has previously been considered to be the main cause of the dyspnoea symptom (10). Nevertheless, a Canadian cohort study concluded that only 28 per cent of the variation in the degree of dyspnoea in patients with mild to moderate COPD could be explained by a variation in FEV1 (9).
Furthermore, FEV1 does not necessarily correlate with the patient’s state of health (11). According to Faull et al., dyspnoea should therefore be screened for and treated in parallel with and independent of lung function (12).
Most common instruments for measuring dyspnoea
The most commonly used instruments for measuring self-reported dyspnoea are the Visual Analogue Scale (VAS), the Numerical Rating Scale (NRS) and various versions of the Borg Scale (13).
In confirmed cases of COPD, diagnosis-specific questionnaires such as the Modified Medical Research Council Dyspnea Scale (mMRC) or the COPD Assessment Test (CAT) are used, both to identify the degree of dyspnoea in the patient and to classify the severity of the condition (14, 15). The mMRC classifies dyspnoea at rest and on exertion on a five-point scale and may also have a prognostic value for COPD (16).
A common feature of all the aforementioned instruments is that they only measure one dimension of dyspnoea: they do not screen for the type of dyspnoea the patient experiences, nor the intensity of dyspnoea or the emotional response invoked by dyspnoea (13).
These factors can impact on how ill or healthy a patient with respiratory problems feels, and which subjective response to treatment is measured (17–19).
An instrument is needed in Norwegian
A multidimensional instrument for self-reported dyspnoea, called the Multidimensional Dyspnea Profile (MDP), has recently been developed and validated in the USA (13). The MDP screens for both the degree and intensity of dyspnoea, distinguishes between different types of breathlessness, and describes the effects of the symptoms on the patient’s mental health (20).
There is currently no instrument in the Norwegian language that, regardless of diagnosis, evaluates multiple dimensions of the patient’s breathing sensations.
A Norwegian version of the MDP has potential for use in clinical practice and research. The purpose of the study was therefore to develop a systematically and linguistically validated Norwegian translation of the MDP.
Method
We translated the original US version of the MDP (13) from English to Norwegian and carried out a linguistic validation in close collaboration with Robert Banzett, who developed the MDP, and the clinical pulmonary department at a local hospital in Norway.
We obtained the original version of the MDP from Mapi, which specialises in the translation and linguistic validation of patient-reported end points (Mapi Research Trust, Lyon, France).
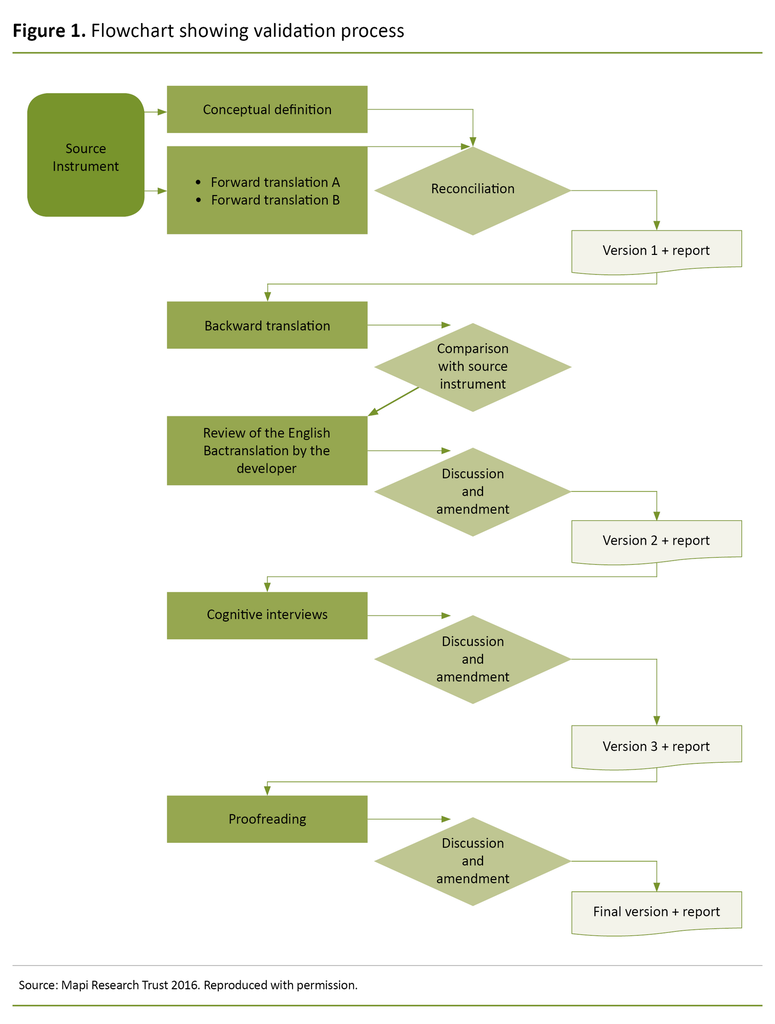
The MDP was translated and linguistically validated in accordance with the Linguistic Validation Guidance of the Multidimensional Dyspnea Profile (Mapi 2016) (Figure 1) and international guidelines (21, 22). We also used the English version of the flowchart in the Norwegian article because it formed the basis for the translation process and for the dialogue with Banzett.
The process involved a translation (steps 1–4), clinical assessment (step 5), patient interviews (step 6), international harmonisation and language editing (step 7).
Translation
(Step 1) The second and third authors, a nurse and a lung specialist respectively, translated the MDP individually from English into Norwegian with a lung specialist with extensive experience from a pulmonary medicine department and outpatient clinic.
(Step 2) The second and third authors compared the translations and developed an agreed version (Norwegian version 1) in collaboration with the first author, who has clinical expertise and research experience with dyspnoea in respiratory patients. All authors are fluent in written and spoken English.
(Step 3) The translated version was then translated from Norwegian to English by a professional translator and a bilingual native English speaker who is fluent in Norwegian.
(Step 4) All the authors compared the translations, and we developed a new, agreed version (Norwegian version 2).
Clinical assessment
(Step 5) Version 2 was then evaluated by the two lung specialists and a pulmonary nurse with many years of experience from a pulmonary outpatient clinic. They provided detailed feedback on whether they considered the key terms to be valid and relevant for patients with dyspnoea (Norwegian version 3).
Patient interviews
(Step 6) In order to ensure that the instrument was clear and understandable to patients, a strategic sample of outpatients with dyspnoea were interviewed and tested using Norwegian version 3. Selection criteria included variation in gender, age and diagnosis (where dyspnoea was the expected symptom).
A pulmonary nurse reviewed each question, and in cases of ambiguity she asked patients to suggest alternative wording. The first author had provided the nurse with the necessary information about using MDP in advance.
The first author participated in the test as an observer and wrote field notes. The nurse sat beside the patient, read the introductory text and questions, and handed the patient the pen to mark their answers on the questionnaire. When the pulmonary nurse saw that the patient was becoming tired, she helped them to mark their answers.
After testing, the patient answered questions about whether the instrument used comprehensible Norwegian, whether the questions were relevant, and whether they understood where to place the crosses (response alternatives: ‘Yes, to a large extent’, ‘Yes, to some extent’ and ‘No, hardly at all’).
We discussed the patients’ feedback and incorporated the necessary changes into a final, linguistically validated translation: Norwegian version 4.
(Step 7) After each translated version, the first author wrote an evaluation report in English, which was included as an assessment basis for international harmonisation. We developed the final Norwegian version of the MDP in collaboration with the copyright holder of the questionnaire and a professional translator.
A pulmonary nurse informed the interviewed patients about the purpose of the study, that participation was voluntary, and that no personally identifiable data would be collected. The Data Protection Officer for research in Nord-Trøndelag Hospital Trust approved the study (case number 2017/4569).
Results
Mapi approved the Norwegian version of the MDP on 9 October 2018, and it is open access on Mapi’s website. Due to copyright, only parts of the questionnaire are presented here (Figure 2). The questionnaire consists of four pages.
A specific time frame or point in time is defined in advance for when the answer relates to. On the first page, a single, general measure of the patient’s breathlessness is requested (A1 scale): ‘Use this scale to rate the unpleasantness or discomfort of your breathing sensations, how bad your breathing feels [felt], using a numeric rating scale (0 = Neutral – 10 = Unbearable)’.
Page two includes five questions about the sensory quality of the breathing sensations (SQ scale): (1) ‘My breathing requires muscle work or effort’ (2), ‘I am not getting enough air or I am smothering or I feel hunger for air’ (3), ‘My chest and lungs feel tight or constricted’ (4), ‘My breathing requires mental effort or concentration’ (5), ‘I am breathing a lot’.
On page three, the patient is asked to rate the intensity of their breathing experience according to a numeric rating scale (0 = ‘None’ – 10 = ‘As intense as I can imagine’) (SQ scale).
On page 4, the patient is asked to indicate how their breathing sensations made them feel (A2 scale): (1) ‘Depressed’, (2) ‘Anxious’, (3) ‘Frustrated’, (4) ‘Angry’ and (5) ‘Afraid’.
Sample and questionnaire
Six outpatients agreed to participate, but one withdrew before the first question was answered. The clinical trial therefore included five patients, two of whom were women. The sample represented the following diagnoses: newly referred breathlessness, asthma, interstitial lung disease, sarcoidosis and COPD.
The median age was 51 years (ranging from 26–67 years). The results showed that everyone ‘to a large extent’ thought that the Norwegian in the questionnaire was comprehensible. Three patients considered the questions relevant ‘to a large extent’, and two patients ‘to some extent’.
When asked if they understood where to place their cross, three responded ‘to a large extent’ and two ‘to some extent’. The median length of time to answer the questions was 7 minutes (between 7 and 8 minutes).
The results showed that everyone ‘to a large extent’ thought that the Norwegian in the questionnaire was comprehensible.
The pulmonary nurse was informed in advance that all text should be read, but for the first two patients she intuitively chose to skip the three lines initially, where the difference between the ‘unpleasantness/discomfort’ and ‘intensity’ of the breathing was explained through an analogy about listening to a radio (Figure 2, page 1).
The difference between these two breathing sensations is exemplified by the sound of a radio: ‘For example, music that you hate can be unpleasant even when the volume is low, and will become more unpleasant as the volume increases; music that you like will not be unpleasant, even when the volume increases.’
The first author emphasised that all text in the questionnaire should be read. One of the patients found it difficult to understand the radio analogy and decided not to complete the questionnaire after the introductory text had been read out.
Discussion
The translation of the MDP into Norwegian resulted in an approved, linguistically validated questionnaire that, regardless of underlying medical conditions, makes it possible to identify the intensity of dyspnoea, distinguish between different types of breathlessness and describe the impact of the symptoms on the patient’s mental health.
The linguistic validation among a sample of outpatients showed that the questionnaire was suitable for use in a clinical context in Norway. The MDP has already been adopted in other countries, and has been translated into Dutch, French, German, Japanese, Portuguese, Turkish and Swedish (23).
In addition, a validation study has been carried out in the original language (24). A Norwegian translation of the MDP can be useful in the assessment, treatment and follow-up of patients with dyspnoea, and in clinical studies.
Linguistic validity, reliability and relevance
Linguistic comprehension and meaning can vary depending on country, culture, time and context (25). We wanted to ensure that the terms used in the US version of the MDP have the same meaning in a Norwegian context.
We therefore carried out a linguistic harmonisation and adaptation in close collaboration with the copyright holder of the MDP, a professional translator and representatives from a clinical pulmonary department. Several terms in the MDP had to be discussed repeatedly before we reached an agreed understanding.
One example was the English word ‘hate’ in the radio analogy (‘music that you hate’) (page 1). ‘To hate’ can be perceived as a strong, negatively charged concept in the Norwegian context and is used differently in the English language. ‘Hate’ was therefore replaced with the Norwegian word for ‘dislike’ (misliker).
Several terms in the MDP had to be discussed repeatedly before we reached an agreed understanding.
Another example was the English word ‘discomfort’ (in connection with the term ‘breathing discomfort’), which was initially translated as ukomfortabel (page 1). However, the way that this word is used in colloquial Norwegian led us to the conclusion that it was more correct to use the word ubehagelig.
In the Norwegian translation of the English statement ‘My chest and lungs feel tight and constricted’, we omitted the word ‘my’. In a Norwegian context, it is not common to use the possessive pronoun in, for example, ‘my lungs’. We therefore thought that it would be more correct to reword the sentence to omit the use of ‘my’.
Finding a translation for the statement ‘I am breathing a lot’ presented a challenge as the present continuous tense is not commonly used in Norwegian, and although the translators agreed to use the present simple Jeg puster mye (‘I breathe a lot’) this is not a common form of expression for this term in colloquial Norwegian. However, a new Swedish study, showed that 18 per cent of patients reported that the infinitive form å puste mye (‘to breathe a lot’) was best for describing their breathing sensations (26).
Norwegian dialects can influence understandings of words and terms
In addition to the two language variants in Norway, there are also a number of dialects, which can impact on the understanding of words and concepts. The Norwegian language is also constantly evolving as a result of, for example, education, social mobility and the use of social media.
It is expected that most Norwegian speakers will be able to understand a questionnaire in the bokmål language variant. The patients in Trøndelag were able to answer all the questions in the MDP.
It is nevertheless conceivable that the pulmonary nurse aided the understanding of the questionnaire by rephrasing the wording orally or providing clarification. She knew the target group well and had experience in instructing patients in filling out questionnaires such as the CAT and the Borg Scale.
In addition to the two language variants in Norway, there are also a number of dialects, which can impact on the understanding of words and concepts.
With the exception of the radio analogy, which can be difficult to understand for people who are perhaps not traditionally radio listeners, we considered the rest of the text and statements in the MDP to be relatively easy to understand.
However, the Norwegian version of the MDP should be tested on a larger patient sample with a variation in age, medical conditions, dialect, socioeconomic status and geographic location.
According to the copyright holder of the MDP, the person administering the MDP for the first time should read the MDP article, including the practical procedure described in the supplementary material (13).
Our experience indicates that, although not a requirement of the MDP copyright holder, training will be required for healthcare personnel who will be guiding the patients through the questionnaire.
Strengths and weaknesses of the study
A strength of the study is that the translation process was carried out in an interdisciplinary group in which the MDP copyright holder also participated. The process was discussion-driven and involved clinicians with experience in patient-centred work and in using questionnaires for self-reporting dyspnoea.
One of the clinicians also had experience in translating St. George’s Respiratory Questionnaire into Norwegian (27). We carried out the translation and linguistic validation systematically and in accordance with accepted international guidelines (21, 22).
Four people participated in the design of the first Norwegian version of the MDP. Linguistic nuances may have been overlooked or interpreted in a different way than was intended in the original version. We have tried to reduce this type of error by involving the copyright holder in the translation process.
A larger number of translators could have been invited to participate, but this may have complicated the process of preparing a final, consensus-based version. Using a professional translator with a non-medical background and testing the questionnaire on patients probably helped to minimise weaknesses in the translation process.
Lack of language comprehension or low levels of proficiency in Norwegian can lead to misunderstandings and loss of distinctions in nuances in the Norwegian MDP version. Standardised training for doctors and nurses who will guide patients in completing the MDP can help reduce such shortcomings.
Patients spent 7 to 8 minutes answering the questionnaire. This extra time may be an additional burden for people who are ill.
The time spent completing the questionnaire is nevertheless considered acceptable when we compare this with the total time spent at a pulmonary outpatient clinic, which usually involves respiratory physiological examinations, X-rays and a doctor’s consultation.
Clinical value of the study
The English version of the MDP was validated in a laboratory setting and in an emergency department in a hospital (24, 28). The questionnaire is relatively easy to use (17, 29) and can be adapted to a reference time frame or point in time for the screening of a patient (dyspnoea now/dyspnoea during the last 24 hours/dyspnoea when the decision was made to contact the accident and emergency unit) (13).
The advantage of the MDP compared to the most commonly used clinical questionnaires for dyspnoea is that it gives quantifiable parameters, whilst also focussing on sensory qualities of dyspnoea and screening for emotional responses to breathlessness, which is important in a holistic approach to patients (30).
For patients who continue to have breathlessness (more than 3) after diagnosis and treatment, the MDP screening can provide more in-depth information than a unidimensional scale and can increase healthcare personnel’s understanding of how different interventions work.
Using the MDP enables healthcare personnel to monitor how well an intervention works, for example: Does the intervention immediately reduce breathlessness, or is the level of anxiety reduced without a reduction in breathlessness?
In doing so, healthcare personnel can capture the complexity of dyspnoea and are better able to tailor the treatment to the individual patient.
Routine screening for dyspnoea
Routine screening for dyspnoea is not common practice in Norwegian hospitals, but it is possible and facilitates the optimisation of care and treatment, including symptom relief (17).
In a study by Beth Israel Deaconess Medical Center in Boston, USA, routine screening and documentation of dyspnoea was introduced for all medical and surgical patients, from the time of arrival and then at each shift throughout the hospital stay (16).
The Boston study used a modified version of the MDP, called the MDP A1 scale, (only one question, see Figure 2, page 1), and after one year, 94 per cent of the nurses reported that it was easy or very easy to perform the dyspnoea screening without affecting the workflow (17).
The MDP may have a clinical value
One weakness of the study is that, to date, there is no scientific evidence for using the MDP as a basis for making clinical decisions. The MDP has not yet been validated for individual patients (13), and more research is needed to establish a measurement for clinically significant differences or changes (31).
Nevertheless, we believe that the MDP has a clinical value because it enables patients to express their subjective experiences with dyspnoea.
Knowledge of the patient’s respiratory sensations and emotional responses (for example, air hunger versus tightness in the chest, anxiety versus depression) is important for assessing how the patient feels here and now, and/or for assessing the effect of a particular intervention or treatment (32).
The MDP can be used both in the acute phase and to follow up patients in a more stable phase (13), and can perhaps also improve the communication between the healthcare personnel in the primary and specialist health services.
We have not tested the MDP in emergency situations here in Norway, but we assume that it would be too time consuming and too much of a burden both for patients and healthcare personnel, with the exception of the first question in the MDP (A1 scale), which is a general measure of dyspnoea. (17).
More research is needed in the Norwegian context
In order to determine the measurement properties of the questionnaire in relation to the intention in a Norwegian context, a validation study is needed, including the testing of reliability and validity in a larger group of informants and in different clinical settings.
The MDP is recommended by an international dyspnoea research network, the Dyspnea Society (33), partly because there is a desire to give more weight to patient-reported outcome measures (19), and because the instrument makes it easier to compare research results between patient categories or medical conditions and between different countries (13, 19).
Conclusion
Through a systematic multi-step method, we have developed and linguistically validated a Norwegian translation of the MDP. This questionnaire for self-reported dyspnoea can be useful in the screening, treatment and follow-up of patients with dyspnoea and in research.
References
1. Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435–52.
2. Langlo NM, Orvik AB, Dale J, Uleberg O, Bjornsen LP. The acute sick and injured patients: an overview of the emergency department patient population at a Norwegian University Hospital Emergency Department. Eur J Emerg Med. 2014;21(3):175–80.
3. Johnson MJ, Currow DC, Booth S. Prevalence and assessment of breathlessness in the clinical setting. Expert Rev Respir Med. 2014;8(2):151–61.
4. Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121(5):1434–40.
5. Abidov A, Rozanski A, Hachamovitch R, Hayes SW, Aboul-Enein F, Cohen I, et al. Prognostic significance of dyspnea in patients referred for cardiac stress testing. N Engl J Med. 2005;353(18):1889–98.
6. Pesola GR, Ahsan H. Dyspnea as an independent predictor of mortality. Clin Respir J. 2016;10(2):142–52.
7. Pesola GR, Argos M, Chinchilli VM, Chen Y, Parvez F, Islam T, et al. Dyspnoea as a predictor of cause-specific heart/lung disease mortality in Bangladesh: a prospective cohort study. J Epidemiol Community Health. 2016;70(7):689–95.
8. Lansing RW, Gracely RH, Banzett RB. The multiple dimensions of dyspnea: review and hypotheses. Respir Physiol Neurobiol. 2009;167(1):53–60.
9. Johnson KM, Safari A, Tan WC, Bourbeau J, FitzGerald JM, Sadatsafavi M, et al. Heterogeneity in the respiratory symptoms of patients with mild-to-moderate COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:3983–95.
10. Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1(6077):1645–8.
11. Murray CJ, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, et al. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608.
12. Faull OK, Marlow L, Finnegan SL, Pattinson KTS. Chronic breathlessness: re-thinking the symptom. Eur Respir J. 2018;51(1).
13. Banzett RB, O'Donnell CR, Guilfoyle TE, Parshall MB, Schwartzstein RM, Meek PM, et al. Multidimensional Dyspnea Profile: an instrument for clinical and laboratory research. Eur Respir J. 2015;45(6):1681–91.
14. Karloh M, Fleig Mayer A, Maurici R, Pizzichini MMM, Jones PW, Pizzichini E. The COPD Assessment Test: What do we know so far? A systematic review and meta-analysis about clinical outcomes prediction and classification of patients into GOLD stages. Chest. 2016;149(2):413–25.
15. Global initiative for Chronic Obstructive Lung Disease. Fontana; 2019. Available at: https://goldcopd.org/gold-reports/ (downloaded 01.11.2019).
16. Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–12.
17. Baker KM, DeSanto-Madeya S, Banzett RB. Routine dyspnea assessment and documentation: nurses' experience yields wide acceptance. BMC Nurs. 2017;16:3.
18. Steindal SA, Torheim H, Oksholm T, Christensen VL, Lee K, Lerdal A, et al. Effectiveness of nursing interventions for breathlessness in people with chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Adv Nurs. 2018;75(5):927–45.
19. Celli BR, Decramer M, Wedzicha JA, Wilson KC, Agusti A, Criner GJ, et al. An official American Thoracic Society/European Respiratory Society statement: research questions in COPD. Eur Respir J. 2015;45(4):879–905.
20. Laviolette L, Laveneziana P, Faculty ERSRS. Dyspnoea: a multidimensional and multidisciplinary approach. Eur Respir J. 2014;43(6):1750–62.
21. Wild D, Grove A, Martin M, Eremenco S, McElroy S, Verjee‐Lorenz A, et al. Principles of good practice for the translation and cultural adaptation process for patient‐reported outcomes (PRO) measures: report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health. 2005;8(2):94–104.
22. Wild D, Eremenco S, Mear I, Martin M, Houchin C, Gawlicki M, et al. Multinational trials – recommendations on the translations required, approaches to using the same language in different countries, and the approaches to support pooling the data: The ISPOR Patient‐Reported Outcomes Translation and Linguistic Validation Good Research Practices Task Force Report. Value Health. 2009;12(4):430–40.
23. Mapi Research Trust. Multidimensional Dyspnea Profile (MDP). Lyon: Mapi Research Trust; 2020. Available at: https://eprovide.mapi-trust.org/instruments/multidimensional-dyspnea-profile (downloaded 29.05.2020).
24. Meek PM, Banzett R, Parshall MB, Gracely RH, Schwartzstein RM, Lansing R. Reliability and validity of the Multidimensional Dyspnea Profile. Chest. 2012;141(6):1546–53.
25. Gjersing L, Caplehorn JR, Clausen T. Cross-cultural adaptation of research instruments: language, setting, time and statistical considerations. BMC Med Res Methodol. 2010;10:13.
26. Ekstrom M, Bornefalk H, Skold M, Janson C, Blomberg A, Sandberg J, et al. Validation of the Swedish Multidimensional Dyspnea Profile (MDP) in outpatients with cardiorespiratory disease. BMJ Open Respir Res. 2019;6(1):e000381. DOI: 10.1136/bmjresp-2018-000381
27. Morland L. Et skjema for måling av helserelatert livskvalitet hos obstruktive lungepasienter – St. George's Respiratory Questionnaire i norsk oversettelse. Lungeforum. 1996;6(1):4–6.
28. Stevens JP, Dechen T, Schwartzstein R, O'Donnell C, Baker K, Howell MD, et al. Prevalence of dyspnea among hospitalized patients at the time of admission. J Pain Symptom Manage. 2018;56(1):15–22.e2.
29. Ekstrom M, Sundh J. Swedish translation and linguistic validation of the multidimensional dyspnoea profile. Eur Clin Respir J. 2016;3:32665.
30. Stevens JP, Sheridan A, Bernstein H, Baker K, Lansing R, Schwartzstein RM, et al. A multidimensional profile of dyspnea in hospitalized patients. Chest. 2019;156(3):507–17.
31. Ekstrom M, Currow DC, Johnson MJ. Outcome measurement of refractory breathlessness: endpoints and important differences. Curr Opin Support Palliat Care. 2015;9(3):238–43.
32. Banzett RB, Moosavi SH. Measuring dyspnoea: new multidimensional instruments to match our 21st century understanding. Eur Respir J. 2017;49(3).
33. Currow DC, Abernethy AP, Allcroft P, Banzett RB, Bausewein C, Booth S, et al. The need to research refractory breathlessness. Eur Respir J. 2016;47(1):342–3.















Comments