Mild cognitive impairment and physical function in community-dwelling older adults
Most community-dwelling older adults had a high function level. However, those with mild cognitive impairment had a significantly poorer grip strength and slower walking speed, and had taken fewer steps.
Background: Mild cognitive impairment (MCI) is a condition that entails reduced cognitive abilities beyond normal aging, but without dementia. Studies support the idea that MCI is associated with physical function and an increased tendency to fall in older people, but most studies concern older people from clinical populations. With regard to risk assessment and prevention, knowledge is also needed on physical function and MCI in community-dwelling older adults in the general population.
Objective: To describe the prevalence of MCI and physical function in community-dwelling older adults, and to compare the physical function level and activity level in community-dwelling older adults with and without MCI.
Method: A cross-sectional study using data collected from 1265 community-dwelling women and men who were aged ≥ 70 years during participation in the population study HUNT4 Trondheim 70+ in the period 2018–2019. We mapped physical and cognitive function using validated measuring instruments. The participants also provided data on grip strength and physical activity measured using an accelerometer.
Results: The age-adjusted scores showed that 17.8 per cent of the women and 21.3 per cent of the men had MCI. The majority of community-dwelling older adults who participated in HUNT4 Trondheim 70+ had a high function level. However, far more women than men in all age categories had scores that indicated an increased risk of functional impairment. Both women and men with MCI had a lower physical function level, poorer grip strength and a slower walking speed, and they walked fewer steps on the most active day compared to those without MCI.
Conclusion: The prevalence of MCI in our sample was lower than in HUNT4 70+, but was in line with findings from several international studies. There was no difference in self-reported impaired function level between participants with and without MCI, but both women and men with MCI had statistically significant lower scores on all function measurements.
Mild cognitive impairment (MCI) appears between normal aging and pathology. MCI involves impaired cognitive function, but not to the extent that the person has lost their independence in relation to daily activities (1). It is estimated that 10–20 per cent of the population aged 65 and over have MCI, and most of them live at home (1).
According to recent figures from the Trøndelag Health Survey, 35.5 per cent of people aged 70 and over have MCI (2). Those with MCI have an increased risk of developing dementia compared to others, and each year about 10 per cent of people with MCI develop dementia (3).
The association between MCI and developing dementia is unclear, however, and this is currently the subject of extensive research. Recent literature reviews also support the idea that grip strength (4), walking speed (5) and physical activity (6) are associated with cognitive function in middle-aged and older people.
Physical activity level has also been posited as one of the main modifiable risk factors for functional impairment and dementia (7). A study that examined changes in cognitive function over a period of 14 years among people aged 65 years and above at the start of the study found a clear correlation between a simultaneous decline in cognitive and physical function (8).
Cognitive function is crucial for carrying out activities in daily life, and it is assumed that performing a variety of activities that challenge the musculoskeletal system can help maintain and improve the cognitive function level.
However, little is known about the prevalence of MCI and the physical function level of community-dwelling older adults in Norway, and the extent of the differences in function level between those with and without MCI. An important goal of Norwegian health policy is for older people to have the opportunity to be as self-sufficient as possible, and to manage by themselves to the greatest extent possible (9).
The need for knowledge about the function level of older people in the general population was emphasised in the Coordination Reform in 2009 (10), and even then the authorities pointed out that ‘[t]he services are marked by insufficient efforts to limit and prevent illness’ (10, p. 13).
According to figures from Statistics Norway, the proportion of the population aged 80 and over will more than triple by 2060 (to 720 000) (11), and by 2050 there will be about three times as many people with dementia as there are today (12).
Having valid and up-to-date knowledge about the health and function level of community-dwelling older adults is crucial for early intervention and municipal planning, as well as for innovation in the education and social care sectors (9, 10).
Objective of the study
The aims of this cross-sectional study are to describe the prevalence of MCI and the physical function level of community-dwelling older adults. In addition, we compare physical function level and physical activity level in community-dwelling older adults both with and without MCI.
Method
Study population
In the autumn of 2018 and the spring of 2019, we invited all permanent residents of a district in Trondheim, a total of 5087 people aged ≥ 70 years, to participate in the study. Participants who for various reasons could not attend the field station were given the option for someone to visit them at home or in a residential institution.
We collected data from questionnaires and various clinical tests using the same protocol as the preliminary study in the fourth Trøndelag Health Survey (HUNT4 70+), which was conducted in the period 2017–2019.
One of the aims of HUNT4 Trondheim 70+ was to study the health of a representative sample in a Norwegian city. The data were collected by health personnel and nursing students in their second year of study at NTNU Trondheim who had certified training. Of the 5087 invited to take part, 1749 chose to participate (34 per cent of those invited).
The sample for this study
Of the 1749 who participated in HUNT4 Trondheim 70+, 1333 reported living in their own flat or house, and we excluded 279 who did not answer this question. We then excluded participants who did not give full answers in the instruments used to map cognitive function and physical fitness (n = 68).
The final sample for the study thus consisted of 1265 participants. Data were missing for some of the individual variables. The number of responses to each of the questions we used is shown in the tables.
Demographic variables
Age is given in number of years. We converted education to a dichotomous variable with either a high or low level of education. The participants in the category with a low level of education had completed lower secondary school, one to two years of upper secondary school, three years of upper secondary school, or had completed an apprenticeship or acquired a journeyman’s certificate.
The participants in the category with a high level of education reported that they had completed less or more than four years of tertiary education. We converted housing status to a dichotomous variable: ‘living alone or living with someone’.
In addition, we measured long-term illness using the following questions from the questionnaire: ‘Do you have any long-term illnesses (lasting at least 1 year), injuries or disorders of a physical or mental nature that impair your function level in daily life?’. We used this as a dichotomous variable with the response alternatives ‘yes’ and ‘no’.
We identified whether the participants had received home nursing care or had been admitted to a nursing home in the past year by asking the following questions: 1: ‘Have you received home nursing care in the last 12 months?’ and 2: ‘Have you been admitted to a nursing home in the last 12 months?’. Both questions were dichotomous with the response alternatives ‘yes’ and ‘no’.
Measuring physical function
To measure physical function, we used the instrument Short Physical Performance Battery (SPPB) (13). SPPB is a validated set of tests used to determine older people’s level of physical function. It has been translated into Norwegian and consists of a balance test, a stand up-sit down test and a four-metre walking test.
Each test is timed, and the result is converted into points (0–4), which in turn are added together to give a total score of between 0 and 12 points. Scores of 9 or lower are associated with functional impairment, vulnerability, need for a place in a nursing home and mortality (13).
The balance test is hierarchical and is divided into three parts: side by side stance, semi-tandem stance and tandem stance. Walking speed is assessed with a four-metre walking test at normal pace, and the best of two measurements is used. Finally, leg strength is assessed using a chair test, where the person being tested is asked to stand up and sit down five times without using their arms. The test is simple, requires little equipment and takes five to seven minutes to complete (14).
The SPPB total is presented both as a continuous variable and as four SPPB categories in line with the categorisation by Guralnik et al. (13) in the original article (0–3, 4–6, 7–9 and 10–12).
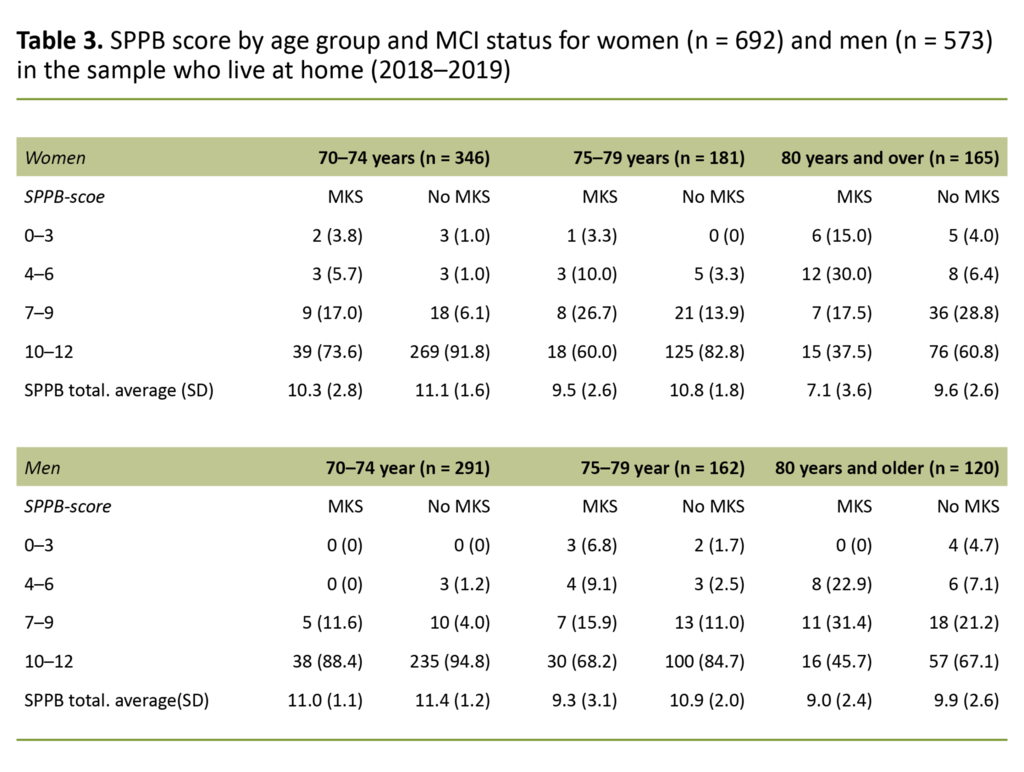
In order to be able to compare our results with findings from the Tromsø study (14), we divided the sample into age groups (70–74 years, 75–79 years, 80 years and over). We measured grip strength in kilograms using a Jamar dynamometer, with three attempts for each hand. We used the best measurement in the analyses.
Objectively measured physical activity
To measure physical activity, we equipped the participants with two accelerometers: one on their lower back and one on their thigh, which they were asked to wear continuously for seven days. The analysis method for this study is based on data from the back sensors. One day is defined as the period from 6 am until midnight. Steps are only registered if a minimum of four are taken consecutively.
We used the Axivity AX3 accelerometer, which has been shown to measure activity with high sensitivity, specificity and accuracy (15). In the results section, we present the number of steps for the day with the most registered steps.
Mapping of cognitive function and MCI
We measured cognitive function using the Montreal Cognitive Assessment Scale (MoCA) (16). The test, which has been translated into Norwegian (17), is designed to measure mild forms of MCI. It measures cognitive capacity in a number of areas: memory, orientation, language, comprehension and visual construction. The maximum score that can be achieved is 30, and a high score indicates better cognitive function. The test takes about 10-15 minutes to complete.
There is no consensus in the literature as to which score should be set as the threshold for MCI. In this study, we chose to use the same age-specific limit values that are used in the guide to HUNT4 Trondheim 70+.
For participants in the age group 70–79 years, the limit value is <22 points, for the group 80–89 years the limit value is <21 and for those ≥ 90 years it is <20. These age-specific thresholds are included in the dichotomous variable MCI (‘yes’ or ‘no’).
Statistical analyses
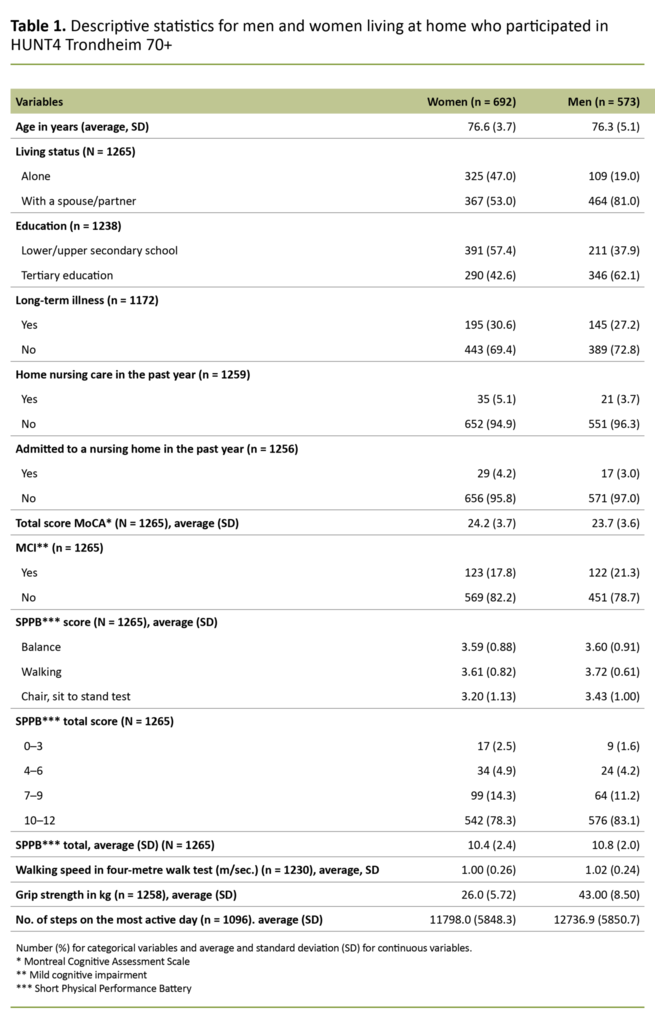
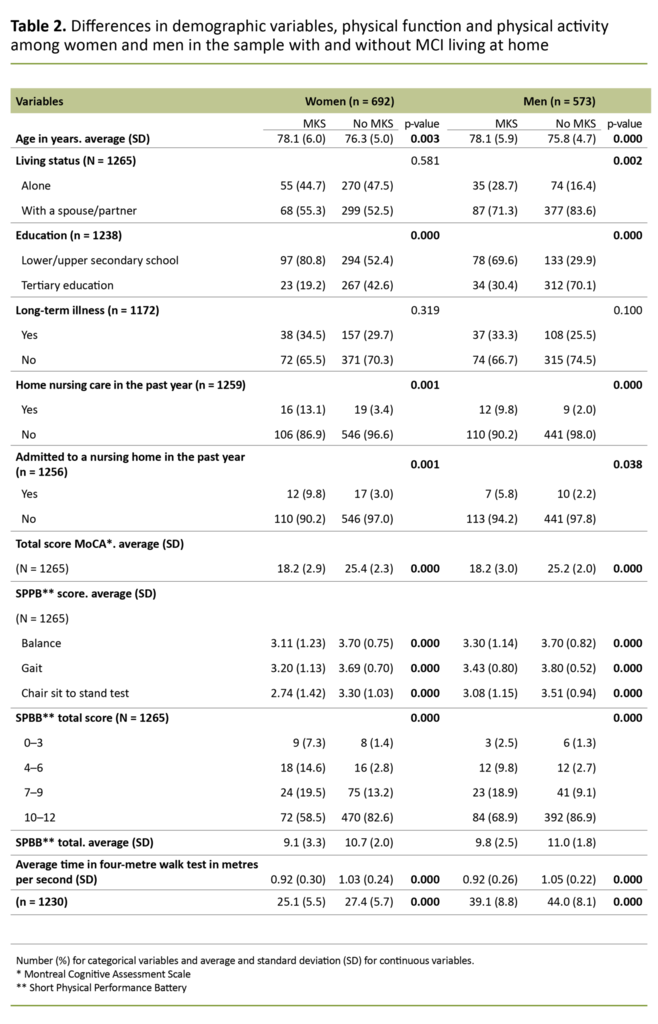
The sample is presented according to gender in Table 1 and the results of univariate analyses are shown in Table 2, where we have compared community-dwelling older adults with and without MCI (broken down by gender). The number and percentage are shown for categorical variables, and continuous variables are presented as averages and standard deviations.
We tested differences between categorical variables using a chi-squared test, and tested differences between continuous variables using a t-test for independent samples. Where the value was expected to be 5 or less in one of the cells in the matrix, we used Fisher's exact test.
In cases where the range was too wide between the groups, we used the Welch test instead of the t-test (18). We chose a significance level of 0.05 for the statistical tests of differences (Table 2). We performed the analyses using the statistical programme IBM SPSS 26.0.
Ethical considerations
All participants have given informed consent for data from the research project HUNT4 Trondheim 70+ to be used in research. This study has been approved by the Regional Committees for Medical and Health Research Ethics (85430/REK-Midt). The data were managed and stored in line with the General Data Protection Regulation (GDPR) and NTNU’s regulations for storing and using medical data.
Results
Of the 1265 participants with complete data from both the MoCA test and the SPPB, 692 were women (54.7 per cent) and 573 were men (45.3 per cent). Demographic variables for the sample are presented in Table 1.
While 47 per cent of the women stated that they lived alone, only 19 per cent of the men stated the same. There were also more women with only a lower or upper secondary education (57.4 per cent) compared with men (37.9 per cent).
About a third of both women and men reported having had a long-term physical or mental illness in the past year that had reduced their function level. Mean SPPB scores were higher for men than women (p = 0.005) (Table 1), with the greatest range among women. This is shown in Figure 1, where we see that more women than men have SPPB scores lower than 10.
Among those aged 80 or over, 60.8 per cent of men and 55.2 per cent of women had a high function level. There was no difference in walking speed between women and men (p = 0.375), but men had taken more steps on the most active day of the measurement period (p = 0.008) and had better grip strength than women (p = 0.000). Comparisons between the groups with and without MCI are shown separately for women and men in Table 2.
There was a statistically significant difference for the vast majority of variables in the comparison between those with and without MCI, both for women and men. For the variable long-term illness, there was no difference between the groups, among either women or men. SPPB scores by age group and MCI status are shown for women in Table 3.
Far fewer of those with MCI than without had a high SPPB score (10–12), and this was the case for both sexes.
Discussion
The aims of this study were to 1) describe the prevalence of MCI and physical function in community-dwelling older adults and 2) compare the level of physical function and physical activity in community-dwelling older adults with and without MCI.
Prevalence of MCI and physical function level
Prevalence of MCI was highest among the men
The prevalence of MCI based on age-adjusted MoCA scores was 17.8 per cent among women and 21.3 per cent among men, and 19.4 per cent overall. These findings support the conclusion in a review from 2014, where 10–20 per cent of those aged 65 years and over met the criteria for MCI (1).
The proportion with MCI in our sample was nevertheless far lower compared with findings from HUNT4 70+, where the prevalence of MCI was 33.0 per cent for women and 38.1 per cent for men (2). This may well be due to the lower average age and higher level of education among the participants in our sample.
The higher prevalence of MCI among men is consistent with findings from several other studies.
The higher prevalence of MCI among men is consistent with findings from several other studies (2, 19, 20). In a Swedish study of 748 older people from the general population, Borland et al. (20) found that old age, gender (male) and a low level of education were predictors of MCI as measured using the MoCA.
However, a relatively recent meta-analysis that included 56 studies concluded that there were no gender differences in the prevalence or incidence of amnestic MCI, but that the prevalence of non-amnestic MCI was higher among women than men (21).
Because our study measured global MCI and did not make a distinction between whether participants had memory impairment (amnestic) or only impairment in other areas of cognition (non-amnestic), it is not possible to determine gender differences in MCI by type of cognitive challenge.
No gender differences in walking speed
Our findings of a lower function level among women than men are consistent with the findings of the Tromsø study (14). The age-related decline in physical function also started earlier in women than in men.
Bergland and Strand concluded that SPPB produces significant ceiling and floor effects. This means that more than 20 per cent of the sample is in the worst or best category (22), and that the SPPB total score, time spent on the chair test or the walking speed gives a better picture of physical function in community-dwelling older adults.
Despite almost 76 per cent of our sample being in the best SPPB category (10–12), we found no gender difference in walking speed in the sample as a whole or within the different age groups. This is consistent with the findings for participants aged 80 and over in the Tromsø study, while men aged 70–79 years walked significantly faster than women of the same age (14).
However, the men had taken more steps than the women on the most active day of the measurement period.
The lack of a gender difference in self-reported long-term chronic illness in our sample may have had a bearing on our finding that there were no gender differences in walking speed among the under 80s. However, the men had taken more steps than the women on the most active day of the measurement period.
One review estimates that healthy older people who adhere to the recommended 30 minutes of daily exercise will take an average of 8000–10 000 steps a day (23). In the sample as a whole, the number of steps on the most active day was almost 12 000 for women and almost 13 000 for men.
As the number of steps on the most active day cannot be compared with studies that give average values for steps during the entire measurement period, we are unable to determine whether the participants in our study adhered to the recommended weekly physical activity.
Number of steps is associated with cognitive function
Both women and men with MCI had statistically significant lower SPPB scores than those without MCI. Far more of the women with MCI had lower SPPB scores (0–6) than the men with MCI. This pattern was also seen in stratified analyses by age group and MCI status.
Walking speed, grip strength and number of steps were also significantly lower in those who had MCI than those who did not.
Walking speed, grip strength and number of steps were also significantly lower in those who had MCI than those who did not. These findings are consistent with a meta-analysis of 26 cross-sectional studies that supported a negative association between various mobility measurements and cognitive function (24).
Recent research also supports an association between number of steps per day and cognitive function, where people in an early phase of MCI take far more steps per day than those with pronounced MCI (25).
Longitudinal studies also find an association between SPPB and a reduction in activities of daily living (ADL), in addition to an increased need for nursing home care and early death (13).
Weak grip strength is a marker of cognitive status
In a review from 2017, the authors concluded that grip strength can be used to observe changes in cognitive function, and that reduced grip strength over time can be a predictor of cognitive impairment in older people (26). This may be because grip strength is correlated with muscle strength (27). Reduced physical function and muscle strength also show a correlation with the number of days spent in hospital and hospital-related deaths (28).
It is nevertheless interesting that we did not find a difference between those with and without MCI in the proportion who reported having had a long-term physical or mental illness that impaired their function in daily living.
There is an association between physical and cognitive function, regardless of clinical status.
This applied to women and men alike. In a Norwegian cross-sectional study in which 98 older patients with newly diagnosed MCI were compared with 115 elderly patients without cognitive impairment, the researchers found that the patients with MCI had a statistically significant poorer performance on three out of six physical tests – physical fitness, the sit to stand test and the six-metre walk test – despite adjustment for demographic factors such as age, level of education and prevalence of disease (29).
This adds to findings from international research that provide support for the association between physical and cognitive function, regardless of clinical status. Nevertheless, it is important to be aware that cognition and physical function levels are not only affected by normal aging processes.
Many different factors can influence test results: nutritional status, drug interactions or adverse effects, infections, metabolic disorders, anaemia, mental health and sleep problems. These factors must be considered for each individual.
Strengths and weaknesses of the study
The strengths of this study are that we used validated instruments and objective measurements of physical and cognitive function in a relatively large group of community-dwelling adults aged ≥ 70.
Weaknesses of the study are the relatively low participation rate and the fact that at present we only have robust maximum values for the number of steps. This makes the study unsuitable for comparison with international literature, where the number of steps in the measurement period is normally stated as average and spread for the entire measurement period.
Another general weakness is that there is no consensus in the literature on which MoCA limit should be used to define MCI. We did not have access to data on who declined the invitation to participate in HUNT4 Trondheim 70+, but since the participation rate was relatively low, we assume that it was the youngest, the healthiest and those with the highest level of education who participated (30).
The proportion who stated that they had a tertiary education was also higher in our sample (50.3 per cent) compared with the participants in HUNT4 70+ (32.3 per cent). The findings from this study cannot therefore be generalised to all Norwegians aged 70 years and over who live at home.
Conclusion
Our survey showed that the prevalence of MCI in this sample was lower than in HUNT4 70+, but was on a par with figures from several international studies.
The majority of community-dwelling older adults who participated in HUNT4 Trondheim 70+ had a high function level, but physical function, grip strength, the walk test and the number of steps on the most active day were considerably lower among those with MCI, both among women and men. Far more women than men in all age categories also had scores that indicated an increased risk of functional impairment.
Use of validated instruments in clinical nursing practice that are not too complex and not too time-consuming can aid the implementation and evaluation of preventive measures and treatment options for community-dwelling older adults.
We would like to thank the Norwegian National Advisory Unit on Ageing and Health, Trondheim local authority and the Trøndelag Health Study (HUNT) for access to data for this study. HUNT is a collaborative project between HUNT Research Centre (Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology – NTNU), Trøndelag county authority, the Central Norway Regional Health Authority and the Norwegian Institute of Public Health. We would also like to thank everyone who participated in HUNT4 Trondheim 70+, as well as the employees and students who contributed to the data collection.
References
1. Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312(23):2551–61. DOI: 10.1001/jama.2014.13806
2. Gjøra L, Strand BH, Bergh S, Borza T, Brækhus A, Engedal K, et al. Current and future prevalence estimates of mild cognitive impairment, dementia, and its subtypes in a population-based sample of people 70 years and older in Norway: the HUNT Study. J Alzheimers Dis. 2021;79(3):1213–26. DOI: 10.3233/jad-201275
3. Roberts RO, Knopman DS, Mielke MM, Cha RH, Pankratz VS, Christianson TJH, et al. Higher risk of progression to dementia in mild cognitive impairment cases who revert to normal. Neurology. 2014;82(4):317–25. DOI: 10.1212/wnl.0000000000000055
4. Carson RG. Get a grip: individual variations in grip strength are a marker of brain health. Neurobiol Aging. 2018;71:189–222. DOI: 10.1016/j.neurobiolaging.2018.07.023
5. Grande G, Triolo F, Nuara A, Welmer A-K, Fratiglioni L, Vetrano DL. Measuring gait speed to better identify prodromal dementia. Exp Gerontol. 2019;124:110625. DOI: 10.1016/j.exger.2019.05.014
6. Turner DT, Hu MX, Generaal E, Bos D, Ikram MK, Heshmatollah A, et al. Physical exercise interventions targeting cognitive functioning and the cognitive domains in nondementia samples: a systematic review of meta-analyses. J Geriatr Psychiatry Neurol. 2020;34(2):91–101. DOI: 10.1177/0891988720915523
7. Orgeta V, Mukadam N, Sommerlad A, Livingston G. The Lancet commission on dementia prevention, intervention, and care: a call for action. Ir J Psychol Med. 2019;36(2):85–8. DOI: 10.1017/ipm.2018.4
8. Chen T-Y, Chang H-Y. Developmental patterns of cognitive function and associated factors among the elderly in Taiwan. Sci Rep. 2016 sep;6(1):33486. DOI: 10.1038/srep33486
9. Meld. St. 15 (2017–2018). Leve hele livet. En kvalitetsreform for eldre. Oslo: Helse- og omsorgsdepartementet; 2017. Available at: https://www.regjeringen.no/contentassets/196f99e63aa14f849c4e4b9b9906a3f8/no/pdfs/stm201720180015000dddpdfs.pdf (downloaded 05.12.2020).
10. St.meld. nr. 47 (2017–2018). Samhandlingsreformen. Rett behandling – på rett sted – til rett tid. Oslo: Helse- og omsorgsdepartementet; 2017. Available at: https://www.regjeringen.no/contentassets/d4f0e16ad32e4bbd8d8ab5c21445a5dc/no/pdfs/stm200820090047000dddpdfs.pdf (downloaded 05.12.2020).
11. Gleditsch RF, Thomas MJ, Syse A. Nasjonale befolkningsframskrivinger 2020. Statistisk sentralbyrå; 2020. Available at: https://www.ssb.no/befolkning/artikler-og-publikasjoner/_attachment/422992?_ts=172798fae98 (downloaded 10.01.2020).
12. Ricci G. Social aspects of dementia prevention from a worldwide to national perspective: a review on the international situation and the example of Italy. Behav Neurol. 2019;8720904. DOI: 10.1155/2019/8720904
13. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. DOI: 10.1093/geronj/49.2.m85
14. Bergland A, Strand BH. Norwegian reference values for the Short Physical Performance Battery (SPPB): the Tromsø Study. BMC Geriatr. 2019;19(1):216. DOI: 10.1186/s12877-019-1234-8
15. Kongsvold AM. Validation of the AX3 accelerometer for detection of common daily activties and postures. NTNU Open; 2016. Available at: https://ntnuopen.ntnu.no/ntnu-xmlui/bitstream/handle/11250/2400440/Masteroppgave_Atle%20Melleby%20Kongsvold.pdf?sequence=1&isAllowed=y (downloaded 21.05.2020).
16. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005 apr.;53(4):695–9. DOI: 10.1037/t27279-000
17. Ihle-Hansen H, Vigen T, Berge T, Einvik G, Aarsland D, Rønning OM, et al. Montreal Cognitive Assessment in a 63- to 65-year-old Norwegian cohort from the general population: data from the Akershus Cardiac Examination 1950 Study. Dement Geriatr Cogn Disord Extra. 2017;7(3):318–27. DOI: 10.1159/000480496
18. Skovlund E. Når bør man velge en ikke-parametrisk metode? Tidsskr Den Nor Legeforening; 2020:16. Available at: https://tidsskriftet.no/2017/05/medisin-og-tall/nar-bor-man-velge-en-ikke-parametrisk-metode (downloaded 22.09.2020).
19. Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, et al. Prevalence of mild cognitive impairment is higher in men. Neurology. 2010;75(10):889–97. DOI: 10.1212/wnl.0b013e3181f11d85
20. Borland E, Nägga K, Nilsson PM, Minthon L, Nilsson ED, Palmqvist S. The Montreal Cognitive Assessment: normative data from a large Swedish population-based cohort. J Alzheimers Dis. 2017;59(3):893–901. DOI: 10.3233/jad-170203
21. Au B, Dale-McGrath S, Tierney MC. Sex differences in the prevalence and incidence of mild cognitive impairment: a meta-analysis. Ageing Res Rev. 2017;35:176–99. DOI: 10.1016/j.arr.2016.09.005
22. Pripp AH. Når målingen går i taket. Tidsskr Den Nor Legeforening. 2019;4. DOI: 10.4045/tidsskr.18.0880
23. Tudor-Locke C, Craig CL, Aoyagi Y, Bell RC, Croteau KA, De Bourdeaudhuij I, et al. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act. 2011;8:80. DOI: 10.1186/1479-5868-8-80
24. Demnitz N, Esser P, Dawes H, Valkanova V, Johansen-Berg H, Ebmeier KP, et al. A systematic review and meta-analysis of cross-sectional studies examining the relationship between mobility and cognition in healthy older adults. Gait Posture. 2016;50:164–74. DOI: 10.1016/j.gaitpost.2016.08.028
25. Chang Y-T. Physical activity and cognitive function in mild cognitive impairment. ASN Neuro. 2020;12:1–9. DOI: 10.1177/1759091419901182
26. Fritz NE, McCarthy CJ, Adamo DE. Handgrip strength as a means of monitoring progression of cognitive decline – a scoping review. Ageing Res Rev. 2017;35:112–23. DOI: 10.1016/j.arr.2017.01.004
27. Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, et al. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281(6):558–60. DOI: 10.1001/jama.281.6.558
28. Cunha AIL, Veronese N, de Melo Borges S, Ricci NA. Frailty as a predictor of adverse outcomes in hospitalized older adults: a systematic review and meta-analysis. Ageing Res Rev. 2019;56:100960. DOI: 10.1016/j.arr.2019.100960
29. Hesseberg K, Bergland A, Rydwik E, Brovold T. Physical fitness in older people recently diagnosed with cognitive impairment compared to older people recently discharged from hospital. Dement Geriatr Cogn Disord Extra. 2016;6(3):396–406. DOI: 10.1159/000447534
30. Langhammer A, Krokstad S, Romundstad P, Heggland J, Holmen J. The HUNT study: participation is associated with survival and depends on socioeconomic status, diseases and symptoms. BMC Med Res Methodol. 2012 sep.;12:143. DOI: 10.1186/1471-2288-12-143











Comments