Prevalence of depressive symptoms and associated factors in people living with HIV in Norway. A cross-sectional study
This study shows that risk factors include living in local authority housing, being infected before the introduction of protease inhibitors, being of Asian, or South or Central American origin, and use of intoxicants.
Background: People living with HIV in Norway have a statutory right to free mental health care. International and Norwegian clinical guidelines for the follow-up and treatment of HIV stress the importance of focussing on mental health in HIV care. No national data exist on the mental health of people with HIV. We therefore wanted to map the occurrence of depressive symptoms in those with HIV, to what extent they received mental health care, and which risk factors for depressive symptoms exist in Norway’s largest HIV cohort.
Method: Over the course of one year, we conducted a cross-sectional study of people over the age of 18 who attended an HIV check-up at the Outpatient Clinic for Infectious Diseases at Oslo University Hospital, Ullevål. Data were collected through structured interviews, from a local HIV quality register (Medinsight.no) and from patient records. All participants completed the Beck Depression Inventory, version II (BDI-II). BDI-II > 14 indicates symptoms of moderate to severe depression.
Results: Of the 486 possible participants, 397 (82%) were included. BDI-II > 14 was found in 76 (19%). Of these, 46 (61%) were not receiving mental health care. Living in local authority housing (odds ratio [OR] 4.0, 95% confidence interval [CI] 1.6–10.3), being infected before the introduction of protease inhibitors (OR 3.2, 95% CI 1.1–7 .9), being of Asian, or South or Central American origin (OR 2.9, 95% CI 1.4–6.0) and use of intoxicants (OR 2.3, 95% CI 1.2–4.3) were risk factors for depressive symptoms. The risk of depressive symptoms was reduced in those receiving antiretroviral therapy (ART) based on non-nucleoside reverse transcriptase inhibitors compared to those using integrase inhibitors (OR 0.5, 95% CI 0.2–0.9).
Conclusion: One in five participants had depressive symptoms, and the proportion not receiving adequate mental health care was considerable. This indicates a need for better mental health care for people living with HIV. We propose that those with one or more risk factors for depressive symptoms be assessed using BDI-II. The possibility of simplifying the screening questions in order to identify depressive symptoms and the extent to which the ART regimen affects depressive symptoms should be further explored.
Introduction
In Norway, approximately 6,500 people are living with HIV, which corresponds to a prevalence of approximately 0.12 per cent (1). With modern antiretroviral therapy (ART), life expectancy for people living with HIV is approximately the same as for the general population (2, 3).
Fully virally suppressed people living with HIV are no longer considered infectious (4), and women can give birth with minimal risk of transmitting the infection to the child (5). HIV infection requires life-long treatment with drugs and must therefore be considered a chronic disease, even in treated patients who are asymptomatic.
Over time, both the chronic infection and ART will increase the risk of, for example, cardiovascular disease, renal failure and osteoporosis (6). The European Aids Clinical Society (EACS) lists a number of factors that impact on the mental health of people with HIV. Old age, use of intoxicants and isolation due to the stigmatisation attached to HIV and AIDS can be a burden for many living with HIV. These may be contributing factors to 20–40% of those living with HIV having depression compared with 7% of the general population (7).
An increased incidence of depression among those living with HIV has also been shown in Sweden (8). The Greater Stockholm HIV Cohort Study was designed to examine the correlation between HIV and, inter alia, depression. Sweden has a national register in which all the diagnosis codes of a person are collated (9–11).
The causal relationship between HIV infection and depression is complex, as both can influence each other. Depression can increase the risk of HIV infection and have a negative effect on compliance with HIV treatment, which in turn can contribute to poorer treatment outcomes and increased morbidity. However, depression can also be a consequence of HIV, both through biological mechanisms and in connection with the practical and psychosocial implications of the diagnosis (9–11).
Well-treated depression has been shown to improve compliance with ART, which underlines the importance of identifying and treating depression in people with HIV (9, 12). EACS therefore emphasises the importance of focusing on mental health in its guidelines for the follow-up and treatment of people living with HIV (7). This is also highlighted in Norwegian guidelines (13).
People with HIV have a statutory right to free mental health care in Norway (14). The absence of national data on the incidence of mental health issues in this patient group is therefore problematic. We believe it is important to have national data, as international data cannot necessarily be transferred to the Norwegian context.
We were inspired by studies on depression and depressive symptoms that had been conducted with Danish HIV cohorts (15–17). The purpose of this study was therefore to survey 1) the prevalence of depressive symptoms among people living with HIV, 2) the proportion with established mental health care, and 3) the risk factors associated with depressive symptoms in order to create a data source for the quality development of care for people with HIV.
Method
Study design
The study is a cross-sectional study of people with HIV who attended a routine check-up at the Outpatient Clinic for Infectious Diseases at Oslo University Hospital (OUS), Ullevål in the period 1 February 2017 to 31 January 2018. Routine check-ups normally take place every six months, alternating between a doctor and a registered nurse (RN). Those who were ≥ 18 years of age and could understand written Norwegian or English could be included, provided they had given written informed consent.
Sample and recruitment
As of 31 December 2018, the Outpatient Clinic for Infectious Diseases at Oslo University Hospital, Ullevål had an HIV cohort of 1,739 people. Of these, some only attended a check-up with a doctor every six months, while others alternated between a doctor and an RN. The doctor with responsibility for the patient determined which option would be most suitable.
Those who also had check-ups with an RN tended to have an uncomplicated HIV infection or a need for frequent follow-up. The appointment letter sent to those invited to see an RN made it clear that mental health would be a priority. The study was conducted within the framework of daily clinical practice, which limited the recruitment sample.
Data collection
After giving written informed consent, the informants were asked about their country of origin, marital status, financial situation, housing situation, use of intoxicants other than alcohol, and follow-up of mental health. Since 2016, these variables have been registered in the local HIV quality register Medinsight.no.
Gender, age, date of diagnosis, mode of transmission, ART regimen, extent of immunosuppression (CD4 count) and viral load per millilitre of blood were obtained from the local HIV quality register and patient records.
Mapping depressive symptoms
To map symptoms of depression, we chose the Beck Depression Inventory, version II (BDI-II) (18). This easily accessible scoring tool for healthcare personnel is part of the Global University depression test (19).
The tool does not require extensive training before use (18) and has a high validity and reliability for depressive symptoms (18). It has also been validated in a Norwegian population (20) and been used in similar studies in HIV populations in Denmark (14–16).
The questionnaire includes 21 groups of statements for measuring the prevalence and severity of depressive symptoms. It covers psychological factors, such as sadness, and physical factors, such as pain. Each topic in the BDI-II has a scaled description with a score from 0–3 points, where 0 = ‘I do not feel sad’ and 3 = ‘I am so sad or unhappy that I can’t stand it’. The person chooses the statement that best describes their state in the preceding two weeks. The higher the score, the stronger the indication of depressive symptoms. The highest possible score is 63.
BDI-II > 14 indicates moderate to severe depressive symptoms and illustrates the need for further investigation. Because depressive symptoms are found in the clinical picture of several mental disorders, it is important to emphasise that the BDI-II maps depressive symptoms with a high validity, keeping in mind the psychiatric competence and other diagnostic tools needed to make a psychiatric diagnosis.
The participants were informed of the result of the screening. In cases of BDI-II > 14, they were offered a referral to a GP for further follow-up and/or a referral to a psychiatrist.
Statistics
We registered the data in SPSS (IBM Statistics for Windows, version 25.0). The data were subsequently double-checked by the RN with primary responsibility for the study.
BDI-II scores were divided into two categories according to the validated threshold for moderate to severe depressive symptoms, i.e. BDI-II > 14 or BDI-II ≤ 14, which indicates normal mood to mild depressive symptoms.
For category variables, reference ranges were defined based on the majority in the study cohort or the most common or most established status in Norwegian society. We divided the variable ‘date of diagnosis’ into two groups, according to the following assessment:
1) Variable: Diagnosed with HIV before protease inhibitors. When protease inhibitors (PI) were introduced in 1995, the impact of HIV on health and life expectancy saw a marked change (21, 22).
2) Variable: Diagnosed withHIV within the past year. A recent HIV diagnosis, within the past year and before inclusion in the study, represents a major strain for the individual and thus a possible risk factor for depressive symptoms.
Descriptive analyses were performed stratified for BDI-II category. We examined risk factors for BDI-II > 14 with univariate and then multivariate logistic regression analysis. The following independent variables were investigated: gender, age, diagnosed with HIV before protease inhibitors, diagnosed with HIV within the past year, mode of transmission, country of origin, marital status, financial situation, housing situation, ART, viral load (the number of virus copies per millilitre of blood), CD4 count (measure of T-cell immune defence) and use of intoxicants.
All independent variables were analysed in a block, where we excluded variables with high multicollinearity (tolerance value ≤ 0.2). In the final adjusted analysis, we included gender, diagnosed with HIV before protease inhibitors, diagnosed with HIV within the past year, country of origin, marital status, housing situation, ART, number of viral copies and use of intoxicants. The result is expressed as an odds ratio (OR) with associated 95% confidence interval (CI), and two-tailed p-value with statistical significance defined as p < 0.05.
Ethical considerations
The overall purpose of the study was to improve the quality of HIV care. The study was supported by the management of the Department of Infectious Diseases at Oslo University Hospital, Ullevål. The department has close, established partnerships with several service user organisations such as HIV Norway and Aksept with a view to ensuring good-quality care for a heterogeneous patient group of vulnerable individuals. As preparation for the study, we held a local service user meeting in December 2016. We informed service users about the study at the meeting, and they gave their support.
The Regional Committee for Medical and Health Research Ethics, South-East Norway (reference number 2016/2210C) granted approval for the study, and the data protection officer at Oslo University Hospital granted approval for storing data (reference number 2016/18515) and keeping the written informed consent forms from the participants in a locked cabinet together with the ID codes for the study.
Results
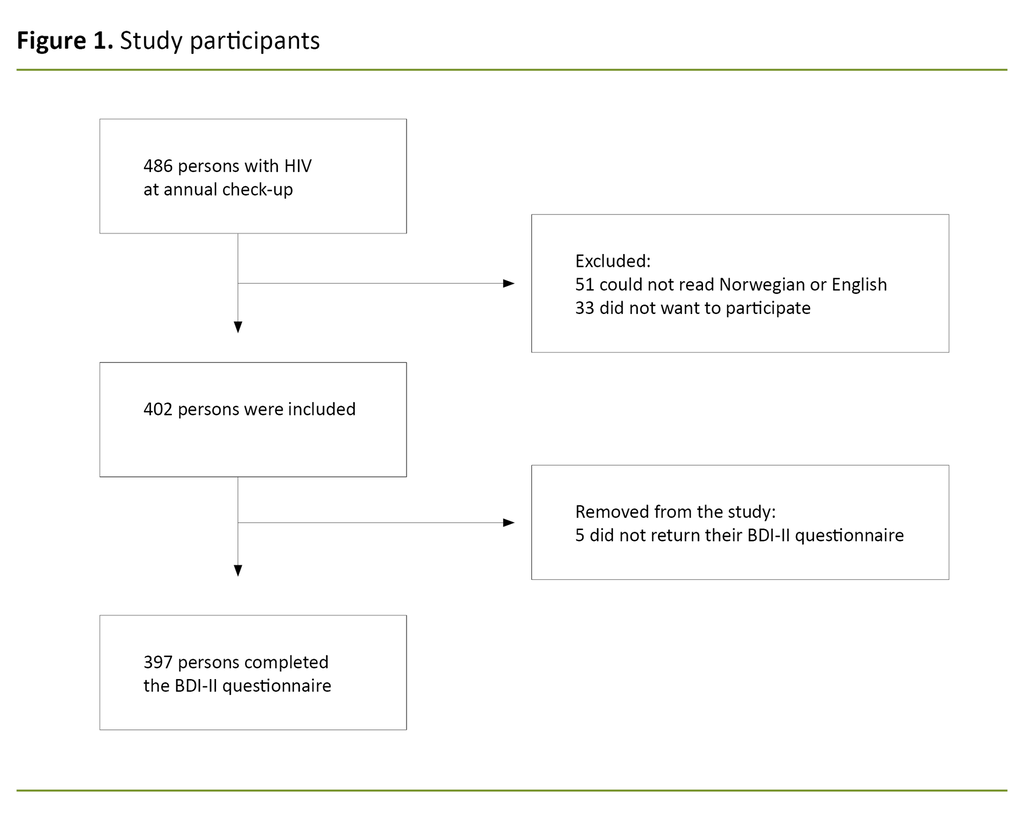
During the inclusion period, 486 people attended a routine check-up with an RN, and 397 (82%) met the inclusion criteria. Reasons for exclusion were language (10%), did not want to participate (7%) and failure to return BDI-II questionnaire within the inclusion period (1%) (Figure 1).
Characteristics of participants
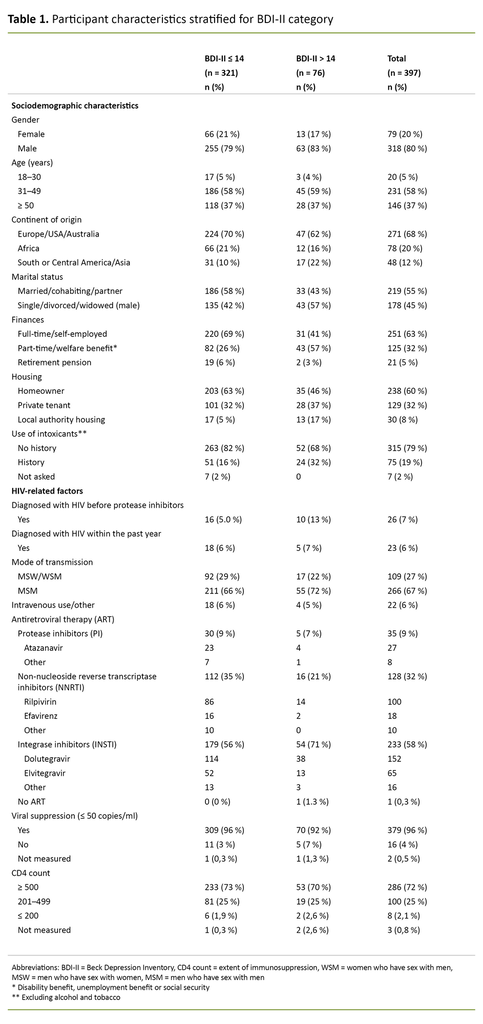
Of 397 people, 80% were men, 58% were between the ages of 31 and 49, 68% were of Western origin, 55% were married, cohabiting or in a relationship, 63% worked full-time, and 60% were homeowners. As many as 79% reported that they had no history of substance use other than alcohol.
In terms of HIV-specific factors, 6% were diagnosed with HIV before the introduction of protease inhibitors, while 6% were diagnosed within the past year. The majority, 88%, were therefore diagnosed after 1995, but before 2016. Men who have sex with men (MSM) made up the largest group of infected people, with 67%.
With the exception of one person, all 397 were receiving ART. The majority of these, 58%, had a regimen that included integrase inhibitors. As many as 95% had viral suppression (< 50 virus copies per ml of blood), and 73% had a CD4 count ≥ 500 (Table 1).
Prevalence of depressive symptoms and proportion receiving mental health care
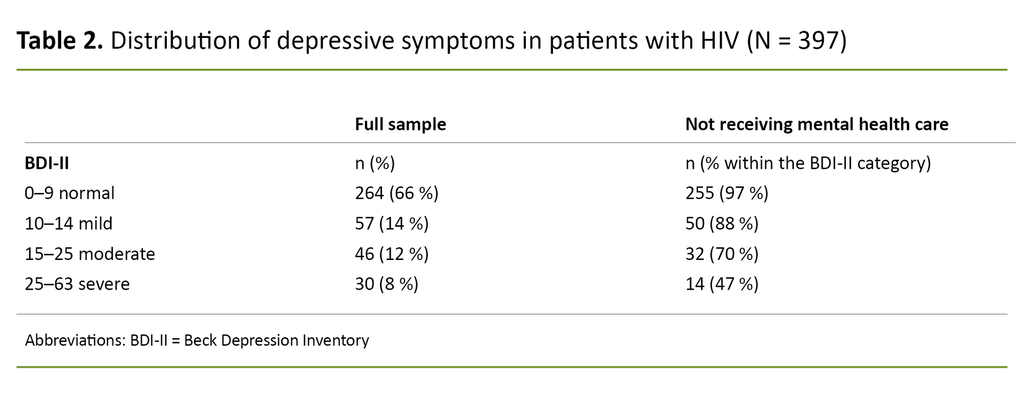
A BDI-II score > 14, which indicates moderate to severe depressive symptoms, was observed in 76 (19%) of participants. Of these, 61% were not receiving mental health care at the time of inclusion.
Even among the 30 with BDI-II scores of 25–63, which indicates severe depressive symptoms, the proportion not receiving mental health care was 47%. People grouped according to the BDI-II categories and the proportion of people not receiving mental health care within these categories are shown in Table 2.
Risk factors for BDI-II > 14 – moderate to severe depressive symptoms
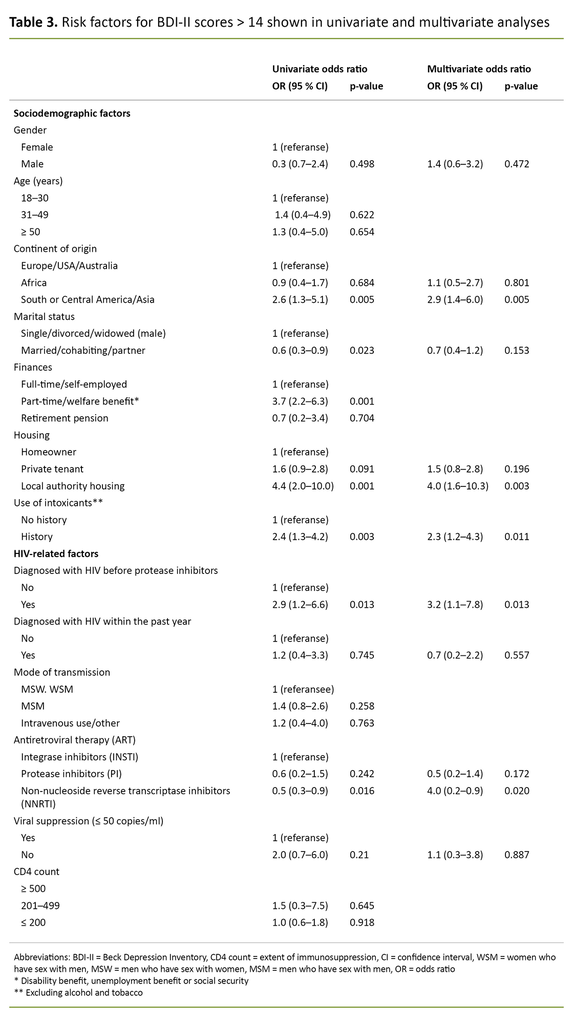
In the multivariate analysis, neither gender nor marital status were associated with BDI-II > 14. The risk of BDI-II > 14 was more than three times higher for participants who were diagnosed with HIV before the introduction of protease inhibitors (OR 3.2, 95% CI 1.1– 7.9), while a diagnosis within the past year was not associated with BDI-II > 14.
Participants of Asian, or South or Central American origin had approximately a three times higher risk of BDI-II > 14 (OR 2.9, 95% CI 1.4–6.0) compared with those from Europe, the United States and Australia. People living in local authority housing had a four times higher risk of BDI-II > 14 (OR 4.0, 95% CI 1.6–10.3) compared to homeowners. A history of substance use – excluding alcohol – also increased the risk of BDI-II > 14 (OR 2.3, 95% CI 1.2–4.3).
Compared with integrase inhibitor-based ART, the risk of BDI-II > 14 was halved in participants with non-nucleoside reverse transcriptase inhibitor-based ART (OR 0.5, 95% CI 0.2–0.9). Virological failure, i.e. > 50 virus copies per millilitre of blood, was not associated with BDI-II > 14 (Table 3).
Discussion
This study showed a 19% prevalence of moderate to severe depressive symptoms in those with HIV. A significant proportion of these, 61%, did not have established mental health care at the time of inclusion. Of the 30 with severe depressive symptoms, the proportion without established mental health care was a worrying 47%.
An HIV diagnosis before the introduction of protease inhibitors, Asian, or South or Central American origin, and use of intoxicants other than alcohol increased the risk of moderate to severe depressive symptoms in adjusted analyses. It was surprising to learn that participants with a non-nucleoside reverse transcriptase inhibitor-based regimen had a lower risk of depressive symptoms compared with those on an integrase inhibitor-based regimen.
The 19% prevalence of depressive symptoms in our data material is lower than the 33–38% found in Danish HIV cohorts using the same scoring tool (15–17). In these studies, participants with a BDI-II > 20 per protocol were offered a consultation with the aim of confirming or ruling out a psychiatric diagnosis, which may represent a possible bias towards higher BDI-II scores.
Although HIV outpatient clinics in Norway lack psychiatric expertise, our experience is that HIV patients establish lasting relationships of trust with the doctor and RN they normally see. Consequently, the HIV outpatient clinics represent a safe arena for conversations and interventions for better physical and mental health. Depressive symptoms identified using the BDI-II tool can include signs of depression, but also other mental disorders (16, 17). We therefore encouraged patients with BDI-II > 14 to contact their GP for further referral, investigation and treatment.
The worryingly high proportion of depressive symptoms in those without established mental health care highlights the need to increase the focus on mental health with a view to improving the quality of HIV care. The study identifies risk factors for BDI-II scores > 14, which can be used for targeted screening and preventive measures. In line with other studies (9, 15–17), we also found that the risk of depressive symptoms increased with poor finances and precarious living conditions.
A comparison of patients from Europe, the United States and Australia showed that the risk of depressive symptoms was higher for those of Asian, or South or Central American origin, but not those from Africa. In a meta-analysis of health among African immigrants, both higher and lower prevalence of mental illness was found compared to non-immigrants.
African asylum seekers report a higher prevalence of mental disorders, and refugees report greater post-traumatic stress (23). In our study, 78 people (20%) were from Africa, but none of these had refugee or asylum seeker status. This finding may explain the low prevalence of depressive symptoms.
The association between substance use and depressive symptoms that has been reported in other Scandinavian (16, 17) and international HIV populations (9) was also confirmed in our study. However, it is worth noting that this relationship has not been found in all HIV populations. This may be due to a combination of several psychosocial factors (24).
To our knowledge, this study is the first to report an increased risk of depressive symptoms among those diagnosed with HIV before the introduction of protease inhibitors. The reason for this is probably complex and linked to life prospects with HIV infection at the time, side effects from first-generation ART and/or HIV-related comorbidities (25).
Some HIV drugs have a negative effect on mental health. The non-nucleoside reverse transcriptase inhibitor efavirenz is associated with a higher risk of suicidal ideation (26) and is not recommended for people with a history of depression (7).
Of 128 patients receiving non-nucleoside reverse transcriptase inhibitor-based ART in this study, only 14% were being treated with efavirenz, with the same proportion among those with BDI-II > 14: 2 out of 16, 12.5%, as those with BDI-II ≤ 14: 16 out of 112, 14.3%.
In five randomised controlled trials, no difference was found in mental health symptoms among participants receiving integrase inhibitor-based ART (dolutegravir and raltegravir), efavirenz (non-nucleoside reverse transcriptase inhibitor) and protease inhibitor-based ART (darunavir) (27).
However, it is important to exercise caution in the interpretation of these results: in line with current guidelines, efavirenz was most likely not given to those with a history of mental illness (7, 13), and other non-nucleoside reverse transcriptase inhibitors with less severe side effects on mental health were not included in the study.
Kolakowska et al. recently summarised the neuropsychiatric side effects of integrase inhibitors. Although standardised registration of neuropsychiatric side effects is challenging and studies on the topic are inconsistent, the data suggest that more caution should be applied to the use of dolutegravir than other integrase inhibitors in patients with mental health challenges until better data become available (28).
In contrast to other studies (9, 10), we found no association between virological response to treatment and depressive symptoms. This finding can most likely be explained by the low proportion of people – 16 – with virological failure (virus load > 50 copies per millilitre of blood) in the study population (Table 1).
The strength of the study was the high participation rate of 82%, compared with 60% in the cited Danish studies (15–17). High participation may have been facilitated by the completion of BDI-II questionnaires during routine check-ups, which enabled participants to get help filling in the questionnaire if necessary.
A long-term relationship of trust with the RN may have contributed to higher participation, but also to an under-reporting of the use of intoxicants: some expressed a concern that sensitive data of this nature would be disseminated to bodies outside the hospital.
The BDI-II questionnaire was only completed once and thus provides a snapshot of depressive symptoms that typically vary over time. This should be taken into account in the follow-up of the individual participants, but it is less important for mapping the prevalence in a population. Unfortunately, the mapping did not include mental disorder history, which is shown to be a clear risk factor for depressive symptoms in the Danish studies (15–17).
The lack of history of mental disorders in this study also complicates the interpretation of the observed association between the BDI-II and ART regimen. It is also a weakness of the study that we did not include the two screening questions for depression, which the HIV guidelines from the European AIDS Clinical Society recommend using every year or every other year: ‘1. Have you often felt depressed, sad or without hope in the last few months?’ and 2. ‘Have you lost interest in activities that you usually enjoy?’ (7).
If the findings in our study are to have relevance for future procedures for following up and treating HIV, we must validate the representativeness of the study cohort of 397 patients in relation to the total HIV cohort, which as of 31 December 2018 contained a further 1,342 patients. Based on the local HIV quality register, which contains prospective sociodemographic, anamnestic and clinical data, we can describe the entire HIV cohort without breaching data protection regulations.
The proportion of men in the study cohort was higher than in the HIV cohort: 80% versus 73%. The proportion of participants aged ≤ 30 was the same (5%), while the group aged 31–49 was overrepresented (58% compared with 44%), and the group aged ≥ 50 was underrepresented (37% compared with 47%).
This study was relatively successful in including people with a non-Western background (32% in the study cohort versus 40% in the HIV cohort). This also applies to people of African origin (20% versus 25%). The proportion of people of Asian, or South or Central American origin was the same (12% in the study cohort versus 13% in the total HIV cohort), which is significant since we found that Asian, or South or Central American origin was a risk factor for BDI-II > 14.
The proportion who were diagnosed with HIV before the introduction of protease inhibitors was small in both cohorts, but underrepresented in the study cohort (7% versus 12% in the HIV cohort). This may indicate a somewhat higher prevalence of depressive symptoms in the total HIV cohort.
Women (20% in the study cohort versus 27% in the HIV cohort) and persons aged ≥ 50 (37% versus 47%) were somewhat underrepresented in the study, but it is uncertain how relevant this finding is, as our study did not identify gender or age as a risk factor for BDI-II > 14.
Conclusion
In this study, 19% of those with HIV had a BDI-II score > 14, which is indicative of moderate to severe depressive symptoms. This finding highlights the need to include a mental health assessment in holistic HIV care. It is alarming that around 50% of those with severe depressive symptoms were not receiving adequate mental health care.
We suggest mapping depressive symptoms using BDI-II in people with HIV at their annual check-up at the Outpatient Clinic for Infectious Diseases. In the event of a lack of resources, the mapping could be limited to patients with one or more risk factors. There is also a need for studies that evaluate the benefit and effect of asking the two screening questions on depression as recommended by the European AIDS Clinical Society (7).
It was surprising that participants receiving non-nucleoside reverse transcriptase inhibitor-based ART had a lower risk of depressive symptoms than those on integrase inhibitor-based ART. This finding indicates a need for more studies to explore the relationship between different ART regimens and mental health. In such studies, it is crucial to identify previous mental health problems as they represent a bias when choosing ART.
The authors declare no conflicts of interest.









Comments