Registered nurses’ knowledge and perceptions of generic substitution
Many registered nurses were unaware that generic substitution can only take place using an approved substitution list, or were uncertain how to use the Norwegian Pharmaceutical Compendium correctly.
Background: Several studies point out that registered nurses (RNs) have inadequate knowledge about drugs. Errors in the healthcare service when handling drugs are also well known. Generic substitution is one of the tasks in drug handling in which errors may arise.
Objective: To gain insight into RNs’ knowledge and perceptions of generic substitution and to assess whether the practice is in accordance with regulations, local routines and the opportunities that exist to check what products can be substituted.
Method: We used an online questionnaire survey of RNs in 23 surgical departments and 28 medical departments at three hospital trusts using Questback software. The data were processed in an Excel spreadsheet, and we used descriptive statistics to describe variables.
Results: Fifty-two per cent were familiar with the hospital’s routines for generic substitution, while 87 per cent knew about the hospital’s substitution list. Only 7 per cent would not consult other sources if they did not find the product in the substitution list. Forty per cent believed that the RN could independently switch between all the drugs registered under the same ATC code in the Norwegian Pharmaceutical Compendium.
Conclusion: Many RNs did not understand that the approved substitution list was required to be the only source for generic substitution. A common misunderstanding was that substitutions could be made on the basis of the ATC register. The RNs needed more knowledge about how to find approved substitutes in the Norwegian Pharmaceutical Compendium.
Several Norwegian studies point to the need for registered nurses (RNs) to have greater medication competence. A qualitative study from 2016 found that there was an unmet need for medication competence in nursing homes, and that instruction in drug handling was not taken seriously enough (1).
Similarly, a questionnaire survey from 2013 concluded that there was a need for greater knowledge and continuing education or courses on drugs and drug handling amongst RNs in nursing homes (2).
Moreover, a doctoral thesis from 2016 concluded that it is necessary to attach greater importance to practical medication competence in nursing education at bachelor’s degree level, and that this competence should be maintained and updated in practice as a registered nurse (3).
Errors and generic substitution
Errors in the use and handling of drugs are well known. A 2007 study of the literature reviewed 35 original articles in the period 1990–2005 about medication-related errors in the health service and concluded that errors arose on average in 5.7 of all the cases where drugs had been given. More than half the errors occurred in connection with the drug handling routine, and one of the risk factors was the inadequate pharmaceutical knowledge of health personnel (4).
RNs encounter generic substitution in both hospitals and nursing homes in that they have to switch between products with different names but with the same active substance and effect. In a 2010 study conducted at a Norwegian hospital, the RNs perceived generic substitution as a risk factor. In their opinion, they had inadequate knowledge to carry out the task, and 42 per cent of the RNs stated that they had experienced errors as a result of generic substitution (5).
A study from a Norwegian nursing home also points to the problem of RNs continually being introduced to new drug products and equivalent drug products, and that it was easy to choose the wrong product (1). Problems linked to generic substitutions are described in a 2011 feature article (6). A search of Pubmed and Sykepleien Forskning did not reveal any other relevant studies dealing specifically with generic substitution.
Requirements for practice
For a long time, statutes and regulations did not deal with RNs’ practice in relation to generic substitution, and when substitution was necessary, there were few options other than comparing information provided in the Norwegian Pharmaceutical Compendium. The 2008 regulations on handling medications, however, set out clear requirements for practice in this area. RNs could only substitute drugs that were approved as interchangeable, and the individual healthcare institutions were required to draw up their own local substitution lists. However, this was limited in practice to products that the Norwegian Medicines Agency had approved as interchangeable.
After 2012, the Norwegian Medicines Agency’s substitution list became directly searchable online, including in the online version of the Norwegian Pharmaceutical Compendium. When the regulations were changed in 2014, this enabled RNs to make generic substitutions directly from the Norwegian Medicines Agency’s list. It is a managerial responsibility to provide written routines for drug handling and to ensure that the staff are familiar with these, as is ensuring that healthcare personnel receive the necessary training and develop their skills in handling drugs (7).
Objective of the study
Safe drug handling requires RNs to have the knowledge required to carry out generic substitution in a safe manner and in line with the regulations. The objective of this study is twofold:
- To gain insight into registered nurses’ knowledge and perceptions of generic substitution.
- To assess whether the practice concurs with the requirements in the applicable regulations and local routines, and with the opportunities available for searching for substitute products.
Method
Design
The study was conducted as an online, personalised questionnaire survey using Questback EFS 10.9 software (8). Some of the questions in the questionnaire comprised Part 1 of the study, which was recently published in the Journal of the Norwegian Medical Association (9). The remaining questions comprised Part 2 of the study and are discussed in this article.
In this part of the study, the questionnaire consisted of 19 questions with fixed responses, 18 of which were obligatory, and 13 questions with open responses, three of which were obligatory. The RNs had to state which hospital trust they worked at and the type of department (medical or surgical) but not which ward. In addition, they stated their age, the number of years they had worked at the hospital, the FTE percentage and type of employment (permanent, deputy or part-time position).
The Likert scale was used for questions on perceptions of generic substitution and how frequently the various versions of the Norwegian Pharmaceutical Compendium were consulted. The RNs were required to respond to seven statements with the alternative responses: ‘agree’, ‘disagree’ or ‘don’t know’.
In Part 1 of the study, the RNs were asked to consider six hypothetical examples of generic substitution (9). They could also comment on how they assessed these substitutions if they so wished. In this part of the study, these supplementary comments were analysed, which entailed identifying misconceptions and then categorising them on the basis of similar content.
Sample
The RNs in the study worked in 23 surgical and 28 medical wards at three different hospital trusts in the Southern and Eastern Norway Regional Health Authority. Those with a main workplace other than a medical or surgical ward were excluded from the study. A local hospital pharmacy provided details of the hospital’s routines.
This showed that all had routines where the RN prepared the drug doses manually for the individual patient based on the medication chart. RNs could also carry out generic substitution based on the substitution list and were required to document the substitution on the medication chart. The hospital trusts each had their own local substitution list. None of the hospitals in the study were university hospitals or used machine packaging of medication in unit doses for the individual hospital patient.
The hospitals used the Tønsys data system for orders from the hospital pharmacy (10). The names and email addresses of RNs who were users of the system in the relevant departments were obtained from Tønsys. The RNs were grouped according to the three hospital trusts and medical or surgical ward.
These six groups were sorted according to ward and then alphabetically according to the name of the RN. I then selected 100 persons from each group using systematic random sampling (11). Contact details were checked against the HR Portal.
Data collection
First of all, I conducted a pilot study with seven RNs in order to optimise the questionnaire. The RNs selected for the study received an information letter in the post a couple of weeks before the questionnaire was distributed. In November 2016, the RNs received information about the study via their work email address, with a link to the questionnaire.
Analysis
The data were transferred from Questback to Excel 2013 for further processing, and descriptive statistics were used to describe variables.
Research ethics considerations
Correspondence with the data protection officer indicated that the study was not subject to notification. It did not contain patient data and thus did not require approval from the Regional Committees for Medical and Health Research Ethics. The responses were anonymised.
Results
Altogether 313 of the RNs completed the questionnaire (52 per cent). Nine of these were excluded from the material, so a total of 304 RNs were included in further analyses. A total of 159 of these worked mainly in a surgical ward and 145 mainly in a medical ward. The number of RNs from the three hospital trusts totalled 103, 84 and 117 respectively.
The mean age was 38 years (range 22–67), the median number of years worked at the hospital was 9 (range 0.5–45), 100 worked in a full-time position (33 per cent), and 290 were permanently employed (95 per cent).
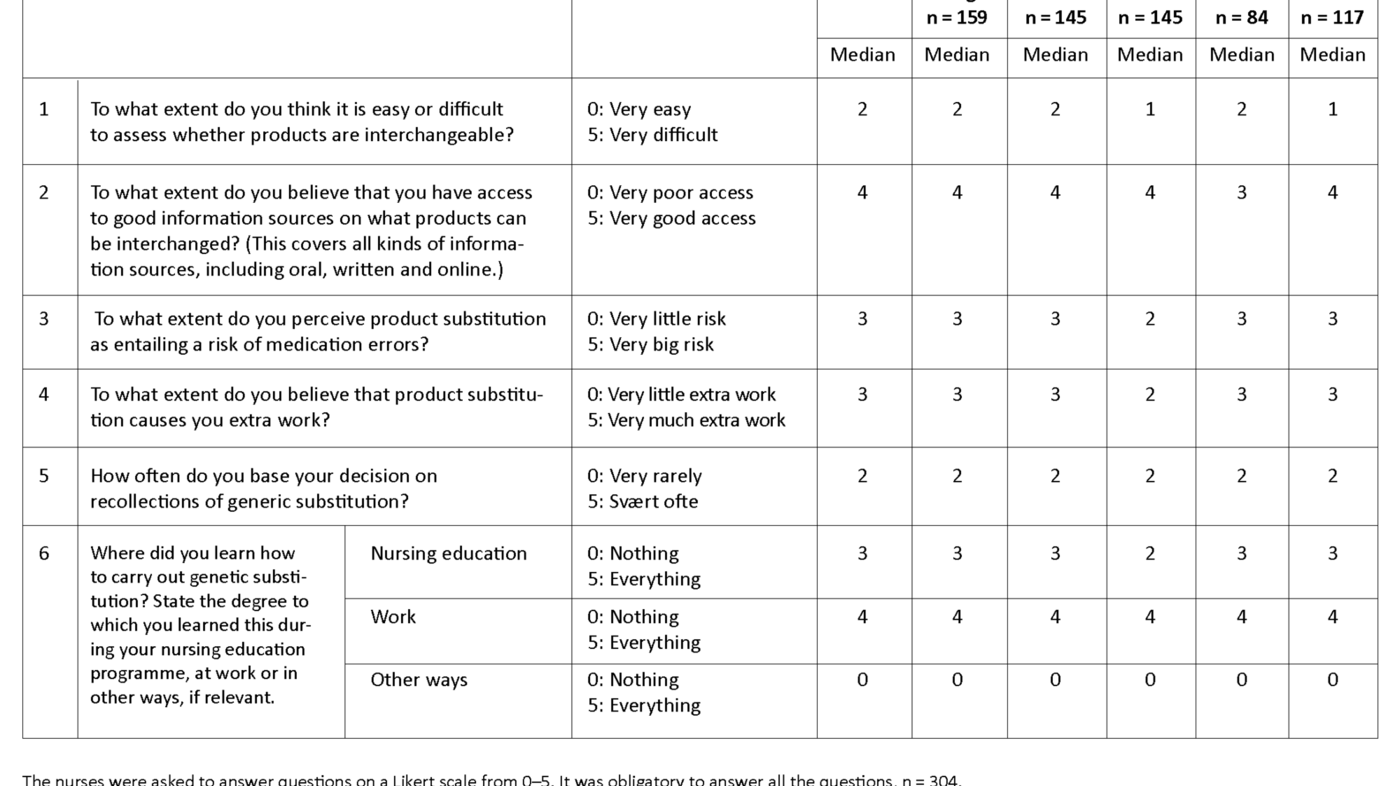
The RNs were asked six questions about generic substitution and they were required to give their views on a Likert scale from 0 to 5 (Table 1). In response to the question on to what extent they thought it was easy or difficult to assess whether drug products are interchangeable, 77 per cent answered either 0 (very easy), or 1 or 2 on the scale.
In response to the question on to what extent they believed they had access to good information sources regarding which products could be substituted, 73 per cent answered either 3, 4 or 5 (very good access). In response to the question on to what extent they had learned during their nursing education programme how generic substitution should be carried out, 37 per cent chose either 4 or 5 on the scale, while the corresponding figure for having learnt this in connection with work was 75 per cent.
In order to gauge how well the RNs knew the hospital’s quality assurance systems, they were asked to respond to seven statements by answering ‘agree’, ‘disagree’ or ‘don’t know’ (Table 2). The answers showed that most of them were familiar with the hospital’s substitution list (87 per cent) while fewer of them (52 per cent) were familiar with the hospital’s written routines for generic substitution.
Question 3 stands out in that only 7 per cent answered in accordance with the regulations on drug handling, while 88 per cent would search further in other sources if they did not find the product on the substitution list.
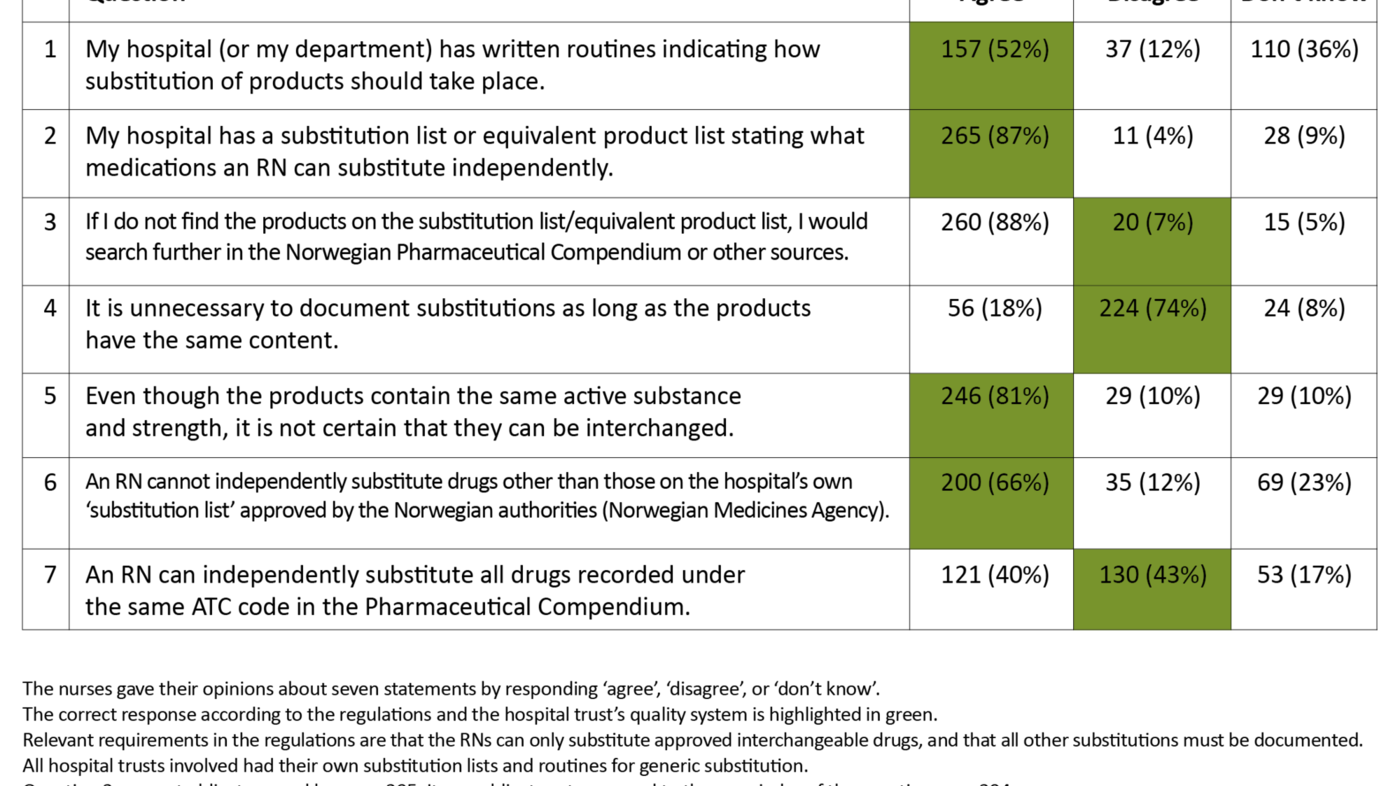
The total number of correct answers in Table 2 (see green highlighting) shows that the RNs answered on average 4.1 of the 7 questions correctly. When analysed in subgroups, the average number of correct answers was 4.2 for surgical departments and 4.0 for medical departments. For the three hospital trusts, the number of correct answers totalled 4.9, 3.4 and 3.8 respectively. Distributed by age groups, the average number of correct answers for the 22–28 age group was 3.8, for the 29–38 age group 4.1, for the 39–51 age group 4.3 and for the 52–67 age group 4.2.
The data were also analysed based on where the RNs had learned how to carry out generic substitution. The 40 who had obtained all their knowledge about carrying out genetic substitution during their nursing education (5 on the scale, see Question 6 in Table 1) answered on average 3.6 of the seven questions correctly, while 107 who had obtained all their knowledge in connection with work (5 on the scale, see Question 6 in Table 1) answered on average 4.4 of the questions correctly.
In the questionnaire, the RNs were asked to give their opinion on six hypothetical examples of generic substitution, stating what information source they used for substitution. The results of this have been published previously (9). Here the RNs could also make supplementary comments if they wished as to how they assessed interchangeability.
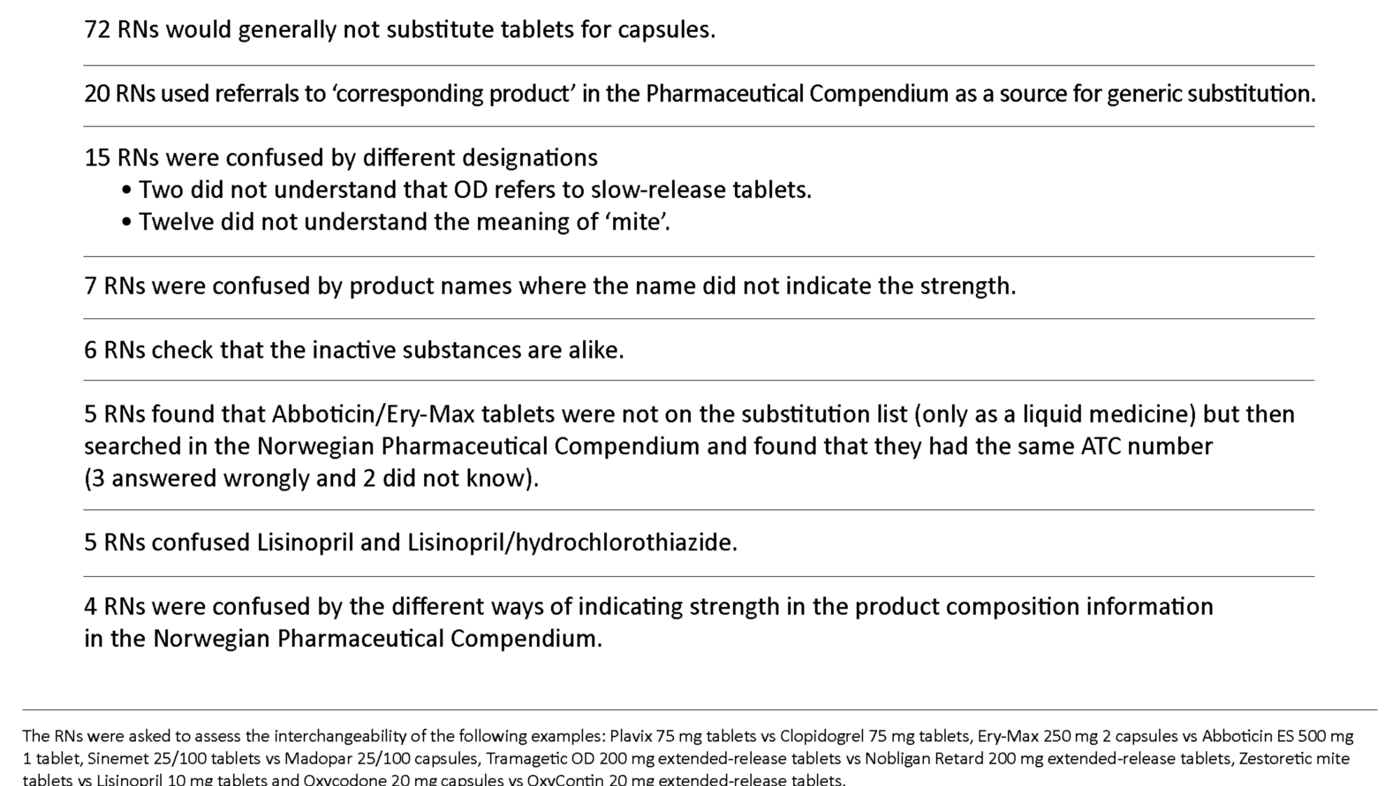
A total of 806 comments were reviewed and analysed to find statements that revealed misunderstandings and errors in the way in which the RNs carried out generic substitution (Table 3). The most frequent misunderstandings were that capsules and tablets generally could not be interchanged, that reference to ‘corresponding product’ in the Norwegian Pharmaceutical Compendium was used as a method for generic substitution, and that there was a variety of problems linked to the understanding of product names.
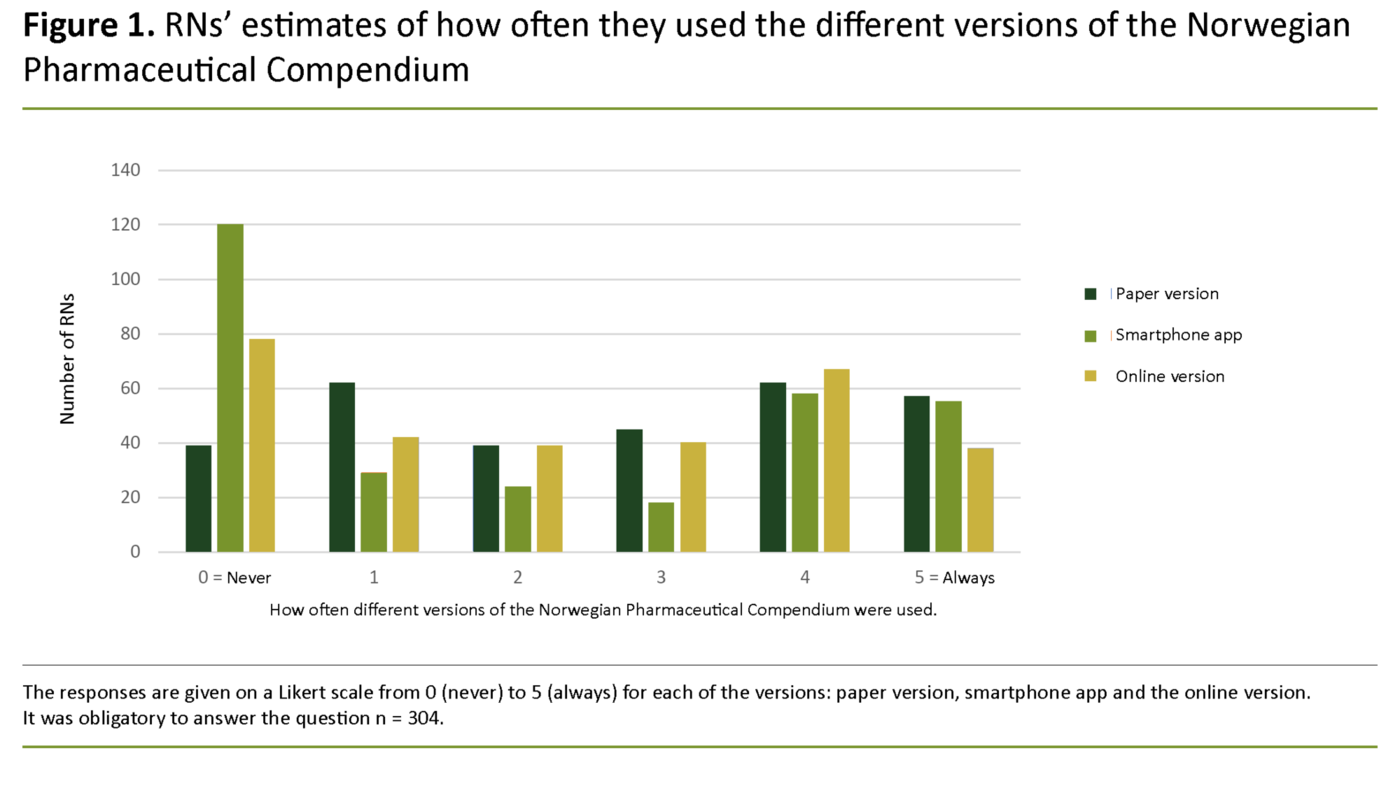
The Norwegian Pharmaceutical Compendium is available in paper and online versions as well as a smartphone app, and there was no great difference in how frequently the various versions were used (Figure 1). Thirty-three out of 304 RNs (11 per cent) never used the Pharmaceutical Compendium, either online or as an app.
Discussion
Perceptions of generic substitution
Many RNs seemed to underestimate the problems related to generic substitution since few of them thought it was particularly difficult to carry this out, and most believed that they had relatively good access to reliable information sources (Table 1). However, Tables 2 and 3 show that many RNs misunderstood a number of factors related to generic substitution, particularly the use of the ATC register.
The first part of this study was based on questions answered at the same time and by the same RNs. In the six hypothetical examples of generic substitution, the median for wrong answers was 2; for example, 23 per cent would substitute extended-release OxyContin tablets with immediate-release Oxycodone capsules.
In the same examples, only 23 per cent acknowledged substitution lists as the only source for substitution. In answer to an open-ended question about how they carried out generic substitution, 62 per cent mentioned specific use of the ATC register (9).
Substitution based on the ATC register
Altogether 40 per cent answered that RNs could independently switch between all medications registered under the same ATC code in the Norwegian Pharmaceutical Compendium, while 17 per cent answered ‘don’t know’ (Table 2). The ATC register is certainly well suited to finding related products but contains no information about what products are approved for substitution (12).
There appeared to be some variation in how thoroughly individuals assessed products in the same ATC group, but most gave the correct response ‘agree’ to the statement ‘Even if the products contain the same active substance and strength, it is not certain that they are interchangeable’.
Use of substitution lists
Altogether 87 per cent had knowledge of the hospital’s substitution list, but 88 per cent would also search in other sources if they did not find products on the substitution list (Table 2). Thus, it appears that many RNs regarded the hospital’s substitution list as one of several sources they could use for substitution rather than being the only approved source for substitution.
Table 3 also indicates that five RNs searched further in the ATC register because they only found Abboticin and Ery-Max as liquid medicine in the hospital’s substitution list, but in this respect only the liquid medicine is approved as interchangeable. The whole point of a substitution list is that it should be the only source used for substitution. Substitutions that are not listed there must be decided by a doctor.
The healthcare institutions themselves decide via their quality assurance systems what substitution lists RNs can use, but only 52 per cent of the RNs knew that their hospital had such a routine. Many RNs relied to some extent on memory in relation to generic substitution (Table 1). Even though you remember products you substitute often, you must be certain that such knowledge originates from an approved substitution list.
Use of the Norwegian Pharmaceutical Compendium
In a questionnaire among RNs in Nord-Trøndelag, just under 30 per cent stated that there was a considerable need for more knowledge about the use of the Pharmaceutical Compendium, while 22.4 per cent had no need for more knowledge (2). This study suggests that there is a considerable need for training in the correct use of the Pharmaceutical Compendium in the case of generic substitution.
In the online version of the Pharmaceutical Compendium, all products with interchangeable alternatives have a link to the ‘substitution group’, i.e. approved packages of the same strength, but this function does not seem to be well known. As of autumn 2017, a new version of the Pharmaceutical Compendium was issued as a smartphone app, where you can search for the same information (13). Most RNs used at least one of these versions to some degree.
Errors and misunderstandings in relation to generic substitution
Misunderstandings can arise in several ways in relation to generic substitution (Table 3) but it is difficult to say how frequently such misunderstandings actually occur since the supplementary comments were given on a voluntary basis. Moreover, the comments were linked to six specific examples of generic substitution, so other examples might have led to completely different results. Table 3 should be regarded, therefore, as providing examples of the kind of misunderstandings that can occur.
Some RNs had the view that capsules generally could not be swapped with tablets. However, immediate-release tablets may well be substituted with immediate-release capsules and extended-release tablets with extended-release capsules. The decisive factor is that the drugs act in exactly the same way in the body.
Some of the copy products refer to ‘corresponding product’ in the Pharmaceutical Compendium because they do not themselves have a complete product description. Some RNs use this information for generic substitution but even though many such products would be approved as interchangeable, this is not necessarily the case.
Some comments show that the product name can be confusing: for example, failure to understand designations such as ‘mite’ and ‘OD’, the fact that the name Zestoretic mite does not indicate the strength of the product, and that similar names may be confused. ‘Mite’ means ‘weak’ and is sometimes used in product names to distinguish between different strengths of the products with the same active substances. OD is an abbreviation for ‘Once Daily’ and means an extended-release tablet or capsule that is to be ingested once daily (24-hour effect). These sources of error show how vital it is to read all product information carefully when undertaking substitution.
Some comments reveal problems distinguishing between active substances and inactive substances, while other informants checked that the inactive substances were the same in cases of generic substitution. However, the inactive substances are normally different, even though the products are approved as interchangeable.
Education, training and knowledge
As mentioned in the introduction, several Norwegian studies show that RNs have too little knowledge about drugs, but this is also a well-known problem internationally (14, 15). In this study, I clearly observe a lack of knowledge about where health personnel are to find the correct information about interchangeable products. In addition, RNs lacked knowledge about forms of medication. In a Norwegian study, RNs in a nursing home called for greater knowledge about drugs and drug handling, highlighting forms of medication and interchangeable drugs in particular (2).
RNs in this study mainly obtained knowledge about generic substitution through work and their nursing education programme, rather than in any other way (Table 1). Whether they obtained knowledge through work or nursing education appeared to have fairly little significance for their level of knowledge, but those who stated that they had obtained their knowledge about generic substitution through their nursing education programme had somewhat fewer correct answers in Table 2 compared with those who had obtained all their knowledge via work.
The youngest age group (22–28 years) had somewhat fewer correct answers than the average. It appears, therefore, that in hospitals and during nursing education many still learn about generic substitution in the way that was common before the introduction of drug handling regulations – although there are no data on what instruction is given in reality.
There were some differences between the three hospital trusts in respect of knowledge about generic substitution, so it is possible that the quality of instruction varied in the different hospital trusts.
Quality improvement
In a project aimed at developing consensus on solutions for risk areas within drug handling at nursing homes and in the community nursing service, generic substitution was regarded as a task where there was a consensus on performing a double check (16). The results of this study also show that generic substitution is a task with considerable risk of error, and that a double check should be performed in accordance with the provisions of the regulations.
In addition, data technology solutions that include the Norwegian Medicines Agency’s substitution list ought to be useful in quality assurance, and two master’s theses (from 2012 and 2013) also find electronic medication charts advantageous for generic substitution (17, 18).
The study shows that many RNs lack vital knowledge about generic substitution. A number of regulations emphasise management’s responsibility for ensuring that staff have the necessary skills (7, 19). Greater use of clinical pharmacists can also provide the healthcare services with greater medication competence. Clinical pharmacists act as advisors in interdisciplinary work, and the Report to the Storting 28 (2014–2015) (white paper on drug handling) highlights clinical pharmacy as an important measure in ensuring correct medication use and greater patient safety (20).
Strengths and weaknesses
A strength of the study is that the RNs were recruited from several hospital trusts and a large number of wards. The greatest weakness is perhaps is that it is very difficult to know how the individual RNs interpret the various questions, and what they emphasise in their responses. The questions could also probably have been formulated more precisely. A qualitative study might have been better suited to answering how the RNs regard generic substitution and why errors occur.
However, a strength was that the results of the second part of the study could be compared with the results of the first part. Therefore, I have been able, for example, to compare how the RNs responded to different statements and what the same RNs specifically did in hypothetical cases of generic substitution.
It would have been useful to have a greater number of more detailed supplementary comments about RNs’ assessments related to generic substitution in the hypothetical examples. A more clearly formulated question with obligatory response on RNs’ assessments in connection with the hypothetical examples of generic substitution would have provided better information about what errors and misunderstandings could occur.
Conclusion
The study shows that many RNs lacked the key knowledge required in relation to generic substitution of drugs. First and foremost, many failed to understand the requirement in the regulations that generic substitution can only take place in accordance with an approved substitution list. The RNs also need better instruction in using the Norwegian Pharmaceutical Compendium correctly. There was a general lack of awareness that the ATC register in the Pharmaceutical Compendium is unsuitable for use in the case of generic substitution and can only be used to find related products.
Most RNs used to some extent either the app or the online version of the Norwegian Pharmaceutical Compendium where the relevant ‘substitution group’, if any, can be found. RNs should also be familiar with the concept of ‘substitution group’ and be aware that this means approved, interchangeable packages. Regardless of the substitution list used, RNs must be aware of the danger of mix-ups when there are different forms of the drug and the names are similar.
References
1. Storli M, Ingebrigtsen O, Nakrem S, Elstad TA. Sikkerhetstiltak for legemidler i sykehjem. Sykepleien Forskning. 2016;11(59801):(e-59801). DOI: 10.4220/Sykepleienf.2016.59801
2. Wannebo W, Sagmo L. Stort behov for mer kunnskap om legemidler blant sykepleiere i sykehjem. Sykepleien Forskning. 2013;8(1):26–34. DOI: 10.4220/sykepleienf.2013.0006
3. Simonsen BØ. Safe medication management. Evaluation and development of medication competence in registered nurses. (Doctor's thesis.) Trondheim: Norges teknisk-naturvitenskapelige universitet, Fakultet for medisin og helsevitenskap, Institutt for klinisk og molekylær medisin; 2016.
4. Krahenbuhl-Melcher A, Schlienger R, Lampert M, Haschke M, Drewe J, Krahenbuhl S. Drug-related problems in hospitals: a review of the recent literature. Drug Saf. 2007;30:379–407.
5. Håkonsen H, Hopen HS, Abelsen L, Ek B, Toverud EL. Generic substitution: a potential risk factor for medication errors in hospitals. Adv Ther. 2010;27(2):118–26.
6. Johansen R. Hvilke legemidler er byttbare? Sykepleien. 2011;99(1):57–9. DOI: 10.4220/sykepleiens.2011.0014
7. Helsedirektoratet. Legemiddelhåndteringsforskriften med kommentarer. Oslo; 2017. Rundskriv IS 7/2015. Available at: https://www.helsedirektoratet.no/rundskriv/legemiddelhandteringsforskriften-med-kommentarer (downloaded 18.11.2019).
8. Questback. Available at: https://www.questback.com/no/ (downloaded 18.11.2019).
9. Johansen R, Andersson Y. Generisk bytte av legemidler i sykehus. Tidsskr Nor Legeforen. 2019;139:36–40. Available at: https://tidsskriftet.no/2019/01/originalartikkel/generisk-bytte-av-legemidler-i-sykehus (downloaded 18.11.2019).
10. Tønsys. Available at: https://tonsys.sahf.no/ebestilling/ (downloaded 18.11.2019).
11. Research Methodology. Systematic sampling. Available at: https://research-methodology.net/sampling-in-primary-data-collection/systematic-sampling/ (downloaded 18.11.2019).
12. WHO Collaborating Centre for Drug Statistics Methodology. ATC. Structure and principles. Available at: https://www.whocc.no/atc/structure_and_principles/ (downloaded 18.11.2019).
13. Statens legemiddelverk. Nytt om legemidler. Oslo; 2018. No. 1/2018. Available at: https://legemiddelverket.no/Documents/Bivirkninger%20og%20sikkerhet/R%C3%A5d%20til%20helsepersonell/NYL/2018/2018_NYL%20nr%201_lavoppl%C3%B8slig.pdf (downloaded 18.11.2019).
14. Sulosaari V, Suhonen R, Leino-Kilpi H. An integrative review of the literature on registered nurses’ medication competence. J Clin Nurs. 2011;20:464–78.
15. Shane R. Current status of administration of medicines. Am J Health-Syst Pharm. 2009;66:42–8.
16. Galek J, Zukrowski M, Grov EK. Slik kan legemiddelhåndteringen bli mer forsvarlig og riktig. Sykepleien Forskning. 2018;13(74117):(e-74117). DOI: 10.4220/Sykepleienf.2018.74117
17. Johnsen A. En sammenlikning av papirbaserte - og elektroniske legemiddelkurver: fordeler, ulemper og effekt på etterlevelse. (Master's thesis.) Oslo: Universitetet i Oslo, Det medisinske fakultet, Institutt for helse og samfunn, Avdeling for helseledelse og helseøkonomi; 2012. Available at: https://www.duo.uio.no/bitstream/handle/10852/30291/MasterxMHAx2012x1.0.pdf?sequence=2&isAllowed=y (downloaded 18.11.2019).
18. Fiske CN, Sjursø ÅM. Elektronisk kurve – mulighet for reduksjon av legemiddelfeil i sykehus? Versjon nr. 1.1. (Masteroppgave.) Universitetet i Agder, Fakultet for helse- og idrettsvitenskap, Institutt for helse- og sykepleievitenskap; 2013. Available at: https://uia.brage.unit.no/uia-xmlui/bitstream/handle/11250/138617/Fiske%20%26%20Sjursoe%20HSI%20%20Masteroppgave%202013%20v%20%201%201.pdf?sequence=1&isAllowed=y (downloaded 18.11.2019).
19. Helsedirektoratet. Veileder til forskrift om ledelse og kvalitetsforbedring i helse- og omsorgstjenesten. Oslo; 2018. IS-2620. Available at: https://helsedirektoratet.no/Retningslinjer/Ledelse%20og%20kvalitetsforbedring.pdf (downloaded 18.11.2019).
20. Meld. St. 28 (2014–2015). Legemiddelmeldingen – riktig bruk – bedre helse. Oslo: Helse- og omsorgsdepartementet; 2015. Available at: https://www.regjeringen.no/contentassets/1e17b19947224def82e509ca5f346357/no/pdfs/stm201420150028000dddpdfs.pdf (downloaded 18.11.2019).









Comments