Safe surgery – set-up and organisation of instrument tables for surgery: a scoping review
Only one guideline detailed a practical and systematic set-up of instrument tables.
Background: Setting up and organising instrument tables is a complex work process that requires a broad evidence base. Adopting evidence-based recommendations improves safety in the operating room.
Objective: The objective of the study was to strengthen the practitioners’ basis for decision-making. This is done through the collection, structuring, summarising and presentation of evidence-based recommendations.
Method: We conducted a scoping review based on Arksey and O’Malley’s methodological framework and Aveyard’s thematic analysis.
Results: The literature is presented under two main topics: 1) Set-up of instruments, equipment and medications on instrument tables, and 2) Reorganisation of instruments, equipment and medications on instrument tables. The recommendations cover prevention of deviations from sterile technique, sharps injuries, retained surgical items in the patient, handling medication and fire safety. One of the guidelines describes a practical, specific and systematic procedure for setting up instrument tables.
Conclusion: The results present a wide range of evidence-based recommendations that can support decision-making in the set-up and organisation of instrument tables. The results of the study can be used in development and improvement work and can form the basis for a standardised set-up of instrument tables for various surgical procedures and for improvements to the ‘Safe Surgery’ checklist. Only one guideline specifies a practical and systematic procedure for setting up instrument tables. We recommend that evidence-based guidelines, standards and clinical procedures be drawn up in Norwegian. We also recommend that operating room nurses’ experiences with setting up and organising instrument tables be examined, and that literature reviews include guidelines from countries that are not covered in this study.
Human error often occurs as a result of deviations from routine practice (1). The World Health Organization (WHO) points out that the knowledge and experience of the surgical team is the most critical resource. WHO also emphasises that implementing standardised safety procedures, such as the ‘Safe Surgery’ checklist, can prevent errors when many people and advanced techniques are involved, thereby improving patient safety (2).
However, WHO also acknowledges that the autonomy of the professions means that there is no standardisation culture in surgical departments (2).
Setting up and organising instrument tables for surgical procedures is a complex work process in which instruments, equipment and medications must be controlled, correctly placed and always available. Organisation helps provide an overview of the instruments and equipment on the instrument tables, and their placement ensures the safety of the patient, surgical team and equipment (3).
In a study conducted at 16 hospitals in Norway, only four surgical departments used written procedures for the set-up of instrument tables (4). This is due to the lack of national and evidence-based guidelines and clinical procedures.
Background
Surgery exposes patients to critical phases where mistakes can be made, leading to injuries and complications (2). Most mistakes are a result of communication failures between members of the surgical team (2, 5, 6). Combined with the lack of guidelines and standards, this can have a negative impact on patient safety.
Reported complications and injuries from surgical procedures include postoperative infections (7), retained surgical items in the patient (5), medication errors (8), injuries from electrical equipment (9), surgical fires (10), transmission of blood-borne pathogens by sharps injuries (11) and infection or disease transmitted by exposure to surgical plume (12).
In any form of surgery, there is a requirement for evidence-based practice (EBP), which encompasses knowledge based on research, practitioners’ experience and the patient’s wishes and needs in the given situation (13). In the study conducted by Hjelen and Sagbakken at three hospitals in Norway, the operating room nurses reported that their work was not evidence-based (14). This highlights the need for literature reviews that simplify access to research-based knowledge for practitioners and help them make informed decisions.
The purpose of this literature study was to generate knowledge that can strengthen practitioners’ basis for decision-making in the set-up and organisation of instrument tables for surgical procedures. We also wanted to improve training and stimulate further research.
The target group is operating room nurses, technicians and nursing students who set up and organise instrument tables, those responsible for drawing up procedures for clinical settings as well as individuals and authorities that develop evidence-based or national guidelines, standards and evidence-based clinical procedures. The study is also aimed at researchers in the field.
We investigated the following research question:
- What evidence-based recommendations are available to support clinical decision-making in the set-up and organisation of instrument tables in the sterile field that contribute to safe surgery?
This article collates, structures, summarises and presents the results of evidence-based recommendations and peer-reviewed studies. The article also highlights research gaps and the need for further research.
Method
Design
The research question was addressed using a five-stage approach in the methodological framework devised by Arksey and O'Malley, which required an accurate and transparent approach without the need for method validation (15).
In addition to the framework, we clarified the research question and the objective of the study, established an effective search strategy and carried out a repeated search and selection process, as recommended by Levac et al. (16).
Furthermore, we have reported the results that are relevant to the research question and discussed implications for future research, practice and policy, as recommended by Levac et al. (16). We started by defining and documenting key concepts, frameworks, objectives and search criteria, and planned the sample as recommended by Peters et al. We then identified research gaps (17).
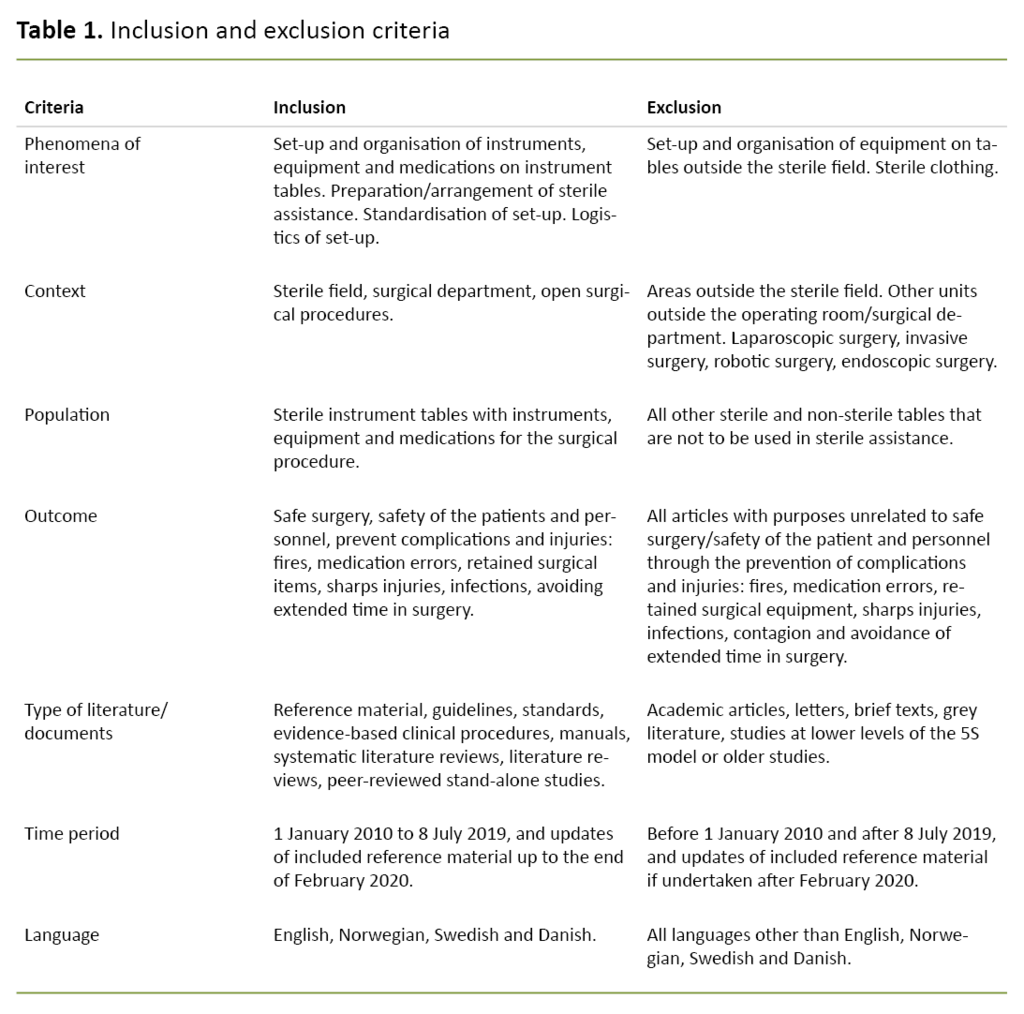
Supplemented by Kahlil et al., the research question helped with the development of specific inclusion and exclusion criteria (18), which in this study provided information on phenomena of interest, population, context, outcome and literature choices (Table 1).
The set-up and organisation of instrument tables is a complex process. This makes a precise, delimited and systematic understanding and assessment difficult, and we therefore chose to carry out a scoping review (18). Earlier literature reviews had not covered this scope.
Identifying the research question (stage 1)
We performed and completed searches in three rounds (in 2017, 2018 and 2019), which generated knowledge about earlier research and helped define the research question and search strategy. The research question focuses on the work process for ‘the set-up and organisation of instrument tables’, which was defined, and keywords selected.
The population was sterile tables with instruments, equipment and medications used in connection with surgical procedures. In the study, ‘instrument table’ is a generic term, and specific terms are ‘Mayo stand’ and ‘back table’.
In addition, instrument trays, instruments, equipment (technical medical equipment, supplementary equipment and disposable equipment such as compresses, surgical covers and swabs) and medications are included. The desired outcome was also specified in the inclusion criteria (Table 1).
Identifying relevant literature (stage 2)
The first and second authors, with input from the third author, planned and developed the literature search using a PICOT form as described by Aveyard (19). Several searches with different terms helped to clarify the inclusion and exclusion criteria as well as the final choice of search terms. The search consisted of text words and subject headings that we discussed thoroughly and adapted to the various databases.
Sources for guidelines and knowledge summaries were reviewed both by searching for overarching search terms or for topics (e.g. operating rooms, safe surgery, patient safety, surgical instruments/equipment, sterile field), and by reviewing content indexes.
This was performed for Norwegian clinical procedures via the Norwegian Electronic Health Library, national guidelines from the Directorate of Health, the Norwegian Electronic Health Library’s guideline database; UpToDate, the pyramid search in the Norwegian Electronic Health Library, Evidence-Based Medicine, Evidence-Based Nursing, NICE, GIN, Socialstyrelsen.se, the Danish Health Authority, the Danish Centre for Clinical Guidelines and the New Zealand Guideline Group: Guideline Central. We also searched the Cochrane Library and Epistemonikos.
The second author performed the final systematic database searches in June and July 2019. The searches were performed in Medline and then in Cinahl, Svemed+ and Embase. The search strategy consisted of text words and controlled search terms that were adapted to the thesaurus of the individual database.
The key search terms we used included the following: operating rooms/theatre/suite/sterile field, surgical instruments/equipment, instrument table, patient safety, safe surgery, guideline/standards/recommendations/checklists. The search terms were combined with the Boolean operators AND and OR. The search strategy from Medline is detailed in an appendix (Appendix 1).
The complexity and scope of the topic resulted in searching for numerous text words and also a recognition that the topic was mainly described in the full text or tables of the articles. It was therefore also necessary to search for general concepts in the individual study databases in addition to resources with more summarised content.
We also carried out repeated manual searches in central organisations’ guidelines: AORN Guidelines for Perioperative Practice: 2018 edition (20), AST Standard of Practice (21), the guidelines from Sweden’s national association for surgical health care (22), WHO Guidelines (23) and EORNA Recommendations (24). In addition to this, we reviewed the references in the individual studies included.
Literature selection (stage 3)
We excluded documents by reading titles, summaries or full text, and selected documents based on the inclusion and exclusion criteria in order to balance feasibility with breadth and scope, as recommended by Peters et al. (17).
Criteria that were included describe phenomena of interest, context, population, outcome, type of literature, time period and language. The search strategy and inclusion of studies were largely governed by their relevance to practice. We included guidelines that we found in manual searches, following discretionary assessments of their relevance to the research question.
In order to quality assure clinicians’ basis for decision-making, as recommended by Peters et al. (17), we deviated from Arksey and O'Malley’s framework by placing an emphasis on the documents’ position in the 5S model (the knowledge pyramid).
Available literature at the top of the pyramid encompassed reference material and guidelines, and at the bottom we find quality-assessed individual studies with original results (25). We selected literature at the top of the pyramid if the recommendations overlapped, and any new knowledge identified lower in the pyramid was also included.
We chose to limit ourselves to ‘open surgery’ as this often requires more equipment and more detailed organisation. In addition, instruments and equipment are mainly set up and organised on the instrument tables, in contrast to invasive endoscopic procedures, where instruments or equipment are also placed in the wound or in special bags in the ‘field’.
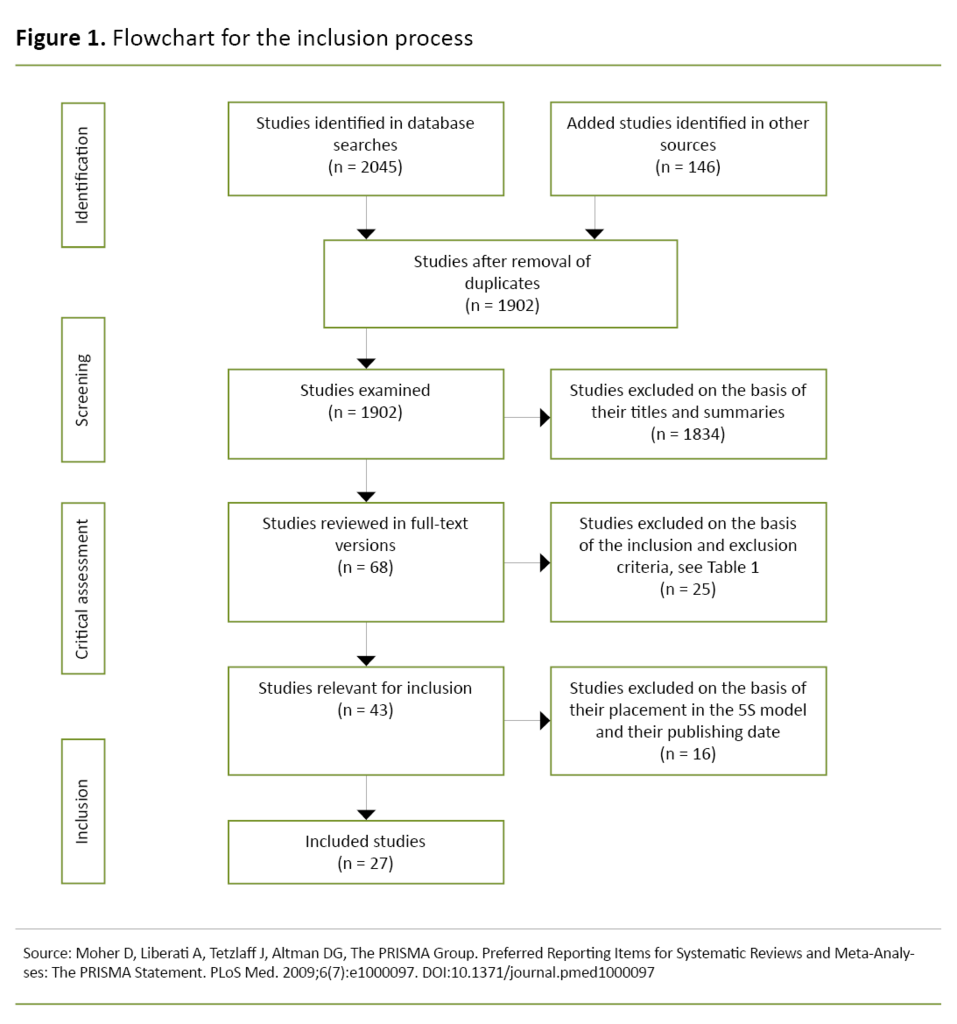
In total, we identified 2191 documents (Figure 1). After removing duplicates in Endnote, the first and third authors individually checked the relevance of the literature in relation to the research question based on the title (first) and summary.
We then compared each other’s selection of documents. Documents that we had both selected were automatically included, and we discussed and assessed the remainder together. The review resulted in a sample of 68 full-text documents. We then individually reviewed all the documents in full text, this time based on the inclusion and exclusion criteria.
After a joint review, both authors agreed that 43 documents met the inclusion criteria. Furthermore, we jointly excluded 16 documents with identical recommendations found in documents higher up the knowledge pyramid.
The final sample consisted of 27 documents based on consensus decisions and agreement through discussion, as recommended by Levac et al. (16) (Figure 1).
Charting the data (stage 4)
The first and third authors presented the selected documents in the form of charts mapping, as recommended by Arksey and O'Malley (15).
Inspired by the categories suggested by Arksey and O'Malley (15), we categorised the documents under author(s), year of publication, document title, journal, country, purpose, type of document/methodology, assessment system and important results. See Appendix 2 [in Norwegian] for details.
Collating, summarising and reporting the results (stage 5)
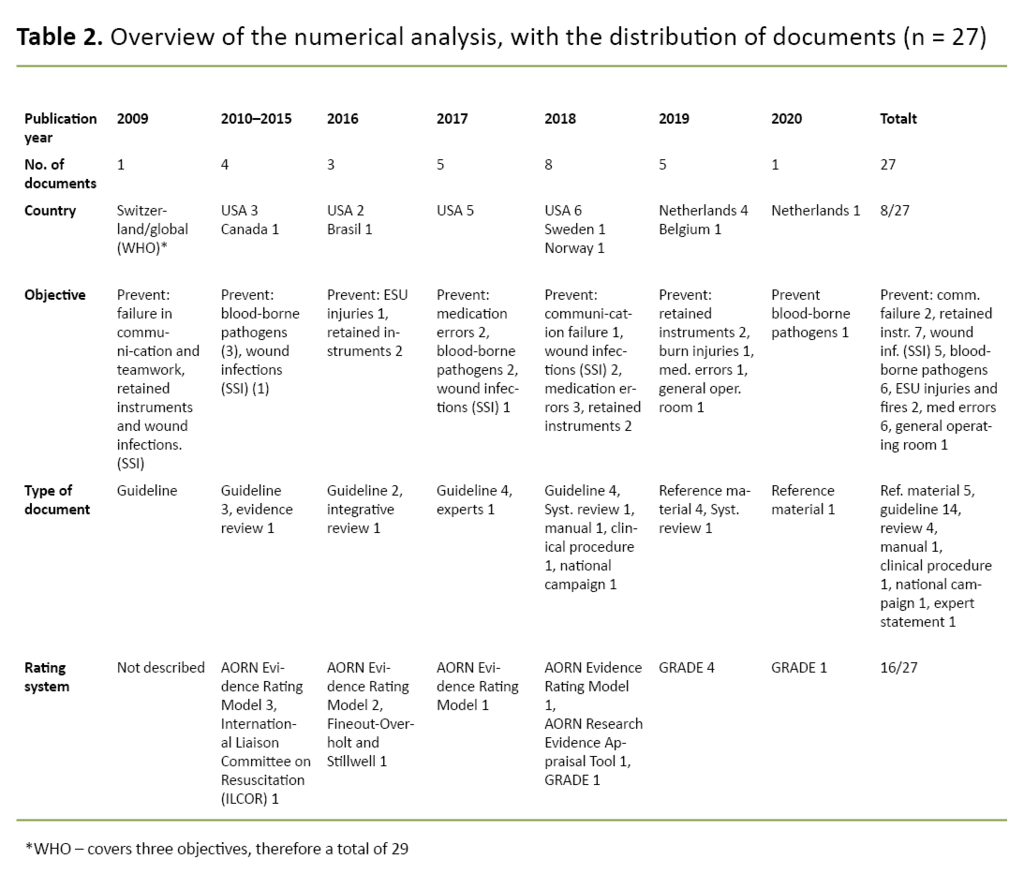
Based on the charts mapping, the first and third authors sorted, summarised, and presented the data in a numerical analysis, as recommended by Arksey and O’Malley (15) (Table 2).
The numerical analysis showed how the documents were distributed between the following categories: year of publication, country, purpose, type of document and assessment system. The WHO Guidelines for Safe Surgery published in 2009 (2) were included because they are still relevant to practice.
The first and third authors performed a thematic analysis as recommended by Aveyard for literature reviews (19), and as recommended by Arksey and O’Malley (15). Our development of themes was based on recommendations that we identified in the selected documents. We then did a broad comparison of the results and ended up with eight preliminary main topics.
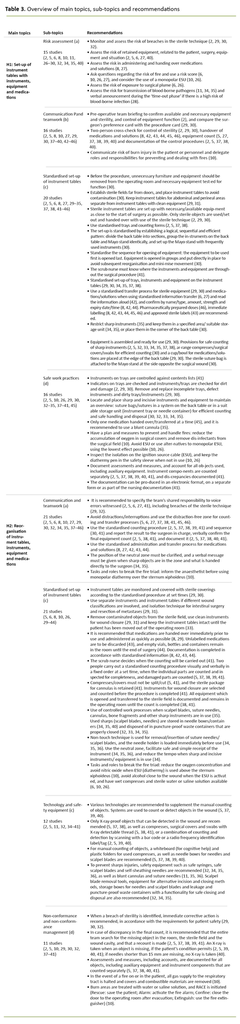
Next, we assessed the topics against the research question. The categorisation and content of the topics were re-systematised and adjusted, and the review resulted in two main topics, each with four sub-topics. We carried out a final review of the results in the charts mapping in order to prevent relevant results in the literature being omitted. The final result is presented in Table 3 and in a narrative presentation.
Results
In summary, the numerical analysis showed that 22 of the 27 documents in the final sample had been published within the last five years. The bulk of them - 16 - were published in the United States, and 9 were from Europe, including 2 from Scandinavia.
The purpose of all the documents was to prevent injury and complications during surgery, mainly aimed at the patient. Two areas; fire safety and transmission of blood-borne pathogens, focused on both patients and staff.
The majority of the documents - 19 - were guidelines or reference material, and 16 documents used an assessment system to grade results or recommendations. See Table 2 for more details.
The thematic analysis resulted in the following:
Theme 1 focused on the initial preparations – set-up of instruments, equipment and medications on instrument tables. The recommendations are limited in time from establishment of the sterile field and the opening of sterile equipment, up until surgery starts subsequent to a time-out phase in line with the ‘Safe Surgery’ checklist.
Theme 2 related to the phase from ‘incision of the skin’ up until the patient is moved out of the operating room, including the final phase in the ‘Safe Surgery’ checklist. The spotlight was on the reorganisation of instruments, equipment and medications on the instrument table based on changes vis-à-vis the patient, surgery and situation.
Set-up of instruments, equipment and medications on instrument tables (H1)
Risk assessment
It is recommended that a risk assessment is performed during the preparations for surgery in order to prevent complications and injuries. The surgical team and the scrub nurse in particular should be focussed on preventing non-compliance with sterile techniques, identifying non-compliance and implementing corrective measures.
Other specific recommendations relate to the risk of transferring and administering medications, assessing the risk (score) associated with the fire triad and the use of monopolar diathermy (Electro Surgery Unit (ESU)), as well as exposure to surgical plume.
The guidelines recommend preventing surgical items being retained in the patient by assessing the risk to the patient (overweight/size, state of health), surgery (trauma, bleeding), the situation (time for preparation and counting) and equipment (quantity, size, detectable). Verbal confirmation is recommended where there is a high risk of blood-borne pathogens. See Table 3, H1:a for details.
Communication and teamwork
Preoperative team briefing and two-person cross-checking are recommended for confirming and controlling that the correct equipment and medications are available. This includes sterility, function, the correct number, as well as checking the surgeon’s preference card and documenting the control routines.
The team must assess and verbally confirm whether there is a fire risk. Furthermore, the team must have a plan to reduce the risk and delegate tasks to prevent and deal with any fire. Observed risk of fire or potential fire must be verbalised loudly and immediately. See Table 3, H1:b for details.
Standardised set-up of instrument tables
The set-up of sterile instruments is described with varying degrees of detail in the documents. The literature recommends using standardised trays with an instrument list, and establishing a logical, sequential and efficient pattern when setting up, which is the same for back tables and Mayo stands.
During dirty surgery, instrument tables are positioned in the room so as to prevent contamination of equipment and instruments. According to recommendations, instrument tables for abdominal and perineal areas must be kept separate, and instruments or equipment that have not been used must remain in specific zones on the back table, separate from those used.
The set-up of sterile instruments is described with varying degrees of detail in the documents.
Instrument tables are established as close to the start of surgery as possible, with sterile equipment only. A standardised transfer process, storage and placing of instruments, equipment and medications is recommended. It is further recommended that information about medication is standardised, doses are pharmaceutically prepared, medication is labelled immediately and that the time factor is heeded in relation to opening and transfer.
The scrub nurse must know where the instruments and equipment are throughout the surgical procedure. Consistent counting is enabled through standardised procedures, and through equipment being assembled and arranged to give an overview and ensure accurate and efficient counting where sharp objects are placed in a specified area on the Mayo stand and back table. See Table 3, H1:c for details.
Safe work practices
The recommendations cover the prevention of non-compliance with sterile techniques and medication errors when opening, handing over and setting up sterile instruments, equipment and medications.
Trays and instruments are controlled against the table of contents and indicators. Their function is also checked and they are examined for dirt. The trays and instruments are replaced if necessary and prepared for use. Only one medication at a time should be handed over, and use of a blunt cannula is recommended.
Sharp instruments and sutures are arranged in sequences designed for efficient and accurate counting as well as safe storage and disposal. Safety measures to break the fire triad are recommended in the form of inspecting the isolation of the ignition source cable (energy-generating equipment) and storing the diathermy pen in an isolated safety sleeve. Consideration should be given to using monopolar diathermy.
Assessments, measures and deviations must be documented. Accounts must also be kept for all items, including supplementary equipment and instrument parts, which are counted separately. Documentation can be produced in an electronic format, on a separate form or as part of the nursing documentation. See Table 3, H1:d for details.
Reorganisation of instruments, equipment and medications on instrument tables (H2)
Communication and teamwork
It is important to note that the team has a joint responsibility for indicating when mistakes are made and when non-compliance with sterile techniques is discovered. In order to prevent interruptions and communication failures, it is recommended that counting and transfer processes are carried out in a distraction-free zone.
A standardised counting procedure is recommended with a fixed order, verbal confirmation and written documentation, as well as a standardised administration and transfer process for medications and solutions. The position of the neutral zone must be clarified in the team, and a verbal message must be given when sharp objects are in use. In order to break the fire triad, the anaesthetist must be notified before monopolar diathermy is used over the sternum. See Table 3, H2:a for details.
Safe work practices
It is recommended that instrument tables are monitored and covered according to a standardised procedure and at set times. In cases of varying wound classifications, separate instruments and instrument tables are recommended, as well as isolation techniques for intestinal surgery or cancer surgery.
Contaminated items are removed from the sterile field, and sterile instruments are used to close wounds. It is recommended that medication is handed over immediately prior to use and administered as quickly as possible. Unlabelled medications are to be discarded. Equipment and waste remain in the operating room until the end of surgery, and documentation is completed in accordance with standardised information.
Two people carry out a standardised counting procedure visually and verbally in a fixed order at a set time. The scrub nurse decides when the counting will be carried out, and all the equipment in the sterile field is included in the final count and documented.
To prevent sharps injuries, controlled work processes are recommended, such as non-touch technique, use of a neutral zone and zones for storage and placement of sharp instruments on the sterile tables. In addition, it is recommended that the tempo is reduced when sharp instruments are in use.
In order to break the fire triad, it is recommended that alcohol is kept away from the wound when energy-generating equipment (diathermy) is in use, that wet compresses and sterile water or saline are available, and that the oxygen concentration is reduced. Nitric oxide should also be avoided when using energy-generating equipment over the sternum. See Table 3, H2:b for details.
Technology and safety equipment
Various technologies are recommended for supporting the manual and systematic counting of items. A combination of manual counting using a whiteboard and plastic folders as well as scanning with a barcode or radio-frequency identification is recommended.
Only X-ray-proof items and compresses, surgical covers and swabs with X-ray detectable thread are used in surgical wounds. Safety equipment such as safety syringes, self-sheathing needles, blunt cannula and suture needles are recommended for preventing sharps injuries. Use of safe scalpel blades is also described.
Scalpel blade removal tools, equipment for alternative incision and closing methods, storage boxes for needles and scalpel blades and puncture-proof waste containers with a functionality for safe closing and disposal are also recommended. See Table 3, H2:c for details.
Non-conformance and non-conformance management
When a breach in sterility is identified, corrective action is recommended immediately where this is compatible with patient safety. Before a surgical procedure is completed, instruments and supplementary equipment need to be counted. If an item is missing, a search must be made of the entire room and in the wound, and a re-count must then be undertaken. If the item is not found, and the patient’s condition permits, an intraoperative X-ray is performed before closing the incision. The result is reported to the surgeon in charge and the team, and assessments, measures and accounts are documented.
If an item is missing, a search must be made of the entire room and in the wound, and a re-count must then be undertaken.
In the event of a fire in or on the patient, all gas to the respiratory tract is stopped. Combustible material is removed, sterile water or saline is applied to fire sites, and the RACE fire procedure is followed (Rescue: save the patient from the fire, Alarm: activate the fire alarm, Confine: close the operating room door after evacuation, Extinguish: use a fire extinguisher during evacuation). See Table 3, H2:d for details.
Discussion
Summary of main findings
In combination, the recommendations relate to teamwork and communication, risk assessment and non-conformance management, standardised set-up, technology and safety equipment as well as safe work practices.
Only one guideline drawn up by operating room technicians sets out recommendations that specify a standardised, systematic and practical set-up of instrument tables. See Table 3 for details.
Status in Norway and potential improvements
The ‘Safe Surgery’ checklist (2) and the EU directive on the prevention of sharps injuries (47), which was introduced to reduce the risk of injuries and complications, are international measures that are important for the set-up and organisation of instrument tables. In addition, evidence-based recommendations help to improve the safety of the operating room and protect patients and staff (6).
Several recommendations found in this literature review are not included in the WHO ‘Safe Surgery’ checklist and should be implemented. The recommendations in this literature review can clarify risk factors that the surgical team should focus on during the time-out phase (H1:1a).
In addition, the team must plan and prepare measures and distribute tasks to prevent and deal with errors and injuries (H1: 1a, 1b and 1d, H2: 2a and 2b), and assess the need to have the necessary safety equipment available (H2:2c).
Several recommendations found in this literature review are not included in the WHO ‘Safe Surgery’ checklist and should be implemented.
An emphasis is placed on the team members’ responsibility to voice any breach of sterility and observed errors, as well as to avoid distractions and interruptions during counting and transfer processes (H2:2a and 2d).
In relation to the closing phase of the ‘Safe Surgery’ checklist, the recommendations provide guidelines for non-conformance management and documentation (H1:1d, H2:2b and 2d). Overall, the recommendations identified can improve the ‘Safe Surgery’ checklist.
There is a lack of Norwegian guidelines and standards for setting up and organising instrument tables. Only one relevant evidence-based clinical procedure for surgical counting is complete and available on the Norwegian Electronic Health Library’s website (41). Prevention of sharps injuries is discussed in the Infection Control Guide (48) and in statutory requirements for the working environment (49).
Written procedures for the set-up and organisation of instrument tables are seldom used in Norway (4). According to the National Health and Hospital Plan 2020–2023, there is considerable variation in many areas of the health service, and the government wants to see more cooperation and sharing of knowledge as well as clinical coordinating committees that will develop procedures (50).
Are evidence-based guidelines and standardised procedures necessary?
The health service is required to apply an evidence-based approach to practice (50). In addition, research shows that using evidence-based recommendations and standardised procedures and techniques improves the safety of processes (2, 6).
Time is critical for patient outcome in all surgical procedures, and according to Høyland et al., planning and preparation save time (51). Accuracy, efficiency and continuity in the surgical team are achieved by following the same pattern and process in line with standardised procedures (52).
One guideline describes a thoroughly considered standardised transfer, storage and placement of instruments, equipment and medications that can contribute to the efficient and safe set-up and organisation of instrument tables (Table 3, H1: 1d).
In Norway, instruments in the sterile field are set up on the basis of a written procedure or picture of the set-up (4), or a ‘separate system for setting up instrument tables’ (53). When an experienced scrub nurse sets up and organises instruments based on a system he or she has developed over time, the work can be performed quickly using the technique he/she has established.
Experienced scrub nurses know that the set-up and organisation of instrument tables must be adapted to different patients and situations, and the work is affected by predictable and unpredictable changes along the way that require continuous reorganisation of equipment and instrument tables. Introducing a standardised set-up that may differ from an established routine can be a daunting prospect for experienced staff.
If the individual scrub nurse has to develop a ‘best practice’ himself/herself, this entails a great deal of responsibility that requires advanced skills. It may also lead to different ‘individual systems’, thereby creating an undesirable variation in patient care and quality. In addition, ‘individual systems’ can lead to challenges when team members are replaced during breaks or in connection with changes to the staff on duty.
If the individual scrub nurse has to develop a ‘best practice’ himself/herself, this entails a great deal of responsibility that requires advanced skills.
When an operating room nurse takes over someone else’s instruments table, it takes time to reorganise it to ‘their own system’. The time and effort required for this work compete with the sterile assistance associated with surgery. Two demanding processes distract each other, and errors often occur in the transitions.
In addition, it is agreed that distractions are one of the main causes of failures and errors, and can result in complications and injuries that must be avoided (5, 6, 39, 45, 46).
Another challenge concerns the training of operating room nurses. Supervisors need to maintain an overview and control in case they have to take over from the student nurse. Students are therefore asked to set up instrument tables using the supervisor’s ‘system’ (53).
If the supervisors have different systems for setting up and organising the instrument tables, it can prevent students from receiving sufficient training and mastering the task. Students are looking for a ‘recipe’ they can learn, and that facilitates better learning (53).
Best practice and effective adaptation to the patient and the situation require evidence-based assessments and skills which in turn require reflective practitioners to have experience and competence. Common procedures based on sustainable knowledge in accordance with evidence-based practice enable operating room nurses to ensure quality in practice to a greater extent and to dispense with unwanted variation.
For students or inexperienced operating room nurses, available guidelines, standards or clinical procedures will provide important support for decision-making.
A common procedure or standard can help ensure that the entire team contributes to patient safety.
Recommendations set out the surgical team’s joint responsibility to notify other team members when errors are observed (2, 5, 6, 33). In order to identify errors, particularly in complex situations like surgery, a common system is required that everyone knows and can monitor, as well as react to when it is not correct. A common procedure or standard can help ensure that the entire team contributes to patient safety.
In addition, the standard or procedure can serve as a quality assurance measure and form a good starting point for adjustments to the patient or the situation. The standard can also remove unwanted variation in the statutory documentation. The documentation plays an important role in quality assuring patient care and students’ learning, and can be used actively in the improvement work in practice.
Assessment of method
The research question in this literature review covers a complex topic that includes literature from many different areas. This may have resulted in errors during the literature search. The results from the search process show that a variation of search terms is required depending on which level in the 5S model the search is aimed at.
In addition, there is also the choice of controlled subject headings and text words, combinations of these and other search engine techniques. This process is complex and time-consuming, and requires researchers to work closely with the librarian. Guidelines that are unavailable in full text, and/or costly to access in full text are further challenges in the process.
In this literature review, it was crucial that the literature was relevant to practice, which resulted in us including various documents. We did not evaluate the methods used in the research literature or rate the quality of the recommendations in the documents, which is in line with Arksey and O'Malley’s framework (15).
However, we also note that 19 documents are at the top of the 5S model, and 16 of these used an assessment system to grade the recommendations.
Inclusion criteria for context (open surgery) and language (English and Scandinavian languages) may have excluded literature that could have contributed new, relevant knowledge. In addition, financial constraints have limited the access to relevant guidelines of surgical nursing organisations in English-speaking countries such as Australia and Canada.
Conclusion
In order to ensure the safety of the patient and staff in connection with surgical procedures, careful planning and preparation of instrument tables is crucial. It is also important to set aside and prioritise time for planning and preparation.
In the literature review, we identified recommendations from a comprehensive and broad area that impact on and are part of choices that are made in the set-up and organisation of instrument tables. The recommendations must be adapted to the patient, the surgery and the surgeon’s preferences for equipment, as well as the team and the situation in general.
The results of the literature review may indicate that there are areas covered by the recommendations that are not included in the ‘Safe Surgery’ checklist, and which are important for patient and staff safety. Only one guideline specified a practical and systematic set-up of instrument tables, drawn up by and aimed at operating room technicians.
We did not identify any evidence-based or national guidelines or standards in Norwegian for the set-up and organisation of instrument tables, and only one relevant clinical procedure for surgical counting in Norwegian is accessible in the Norwegian Electronic Health Library.
Implications for clinical practice, training and research
Evidence-based recommendations can help staff to adopt an evidence-based approach to practice as they serve as a support for decision-making among operating room nurses, technicians and nursing students who set up and organise instrument tables, and are important for the development of clinical procedures in hospitals.
The lack of guidelines and standards should be considered problematic and the medical profession in Norway should be encouraged to develop evidence-based or national guidelines and standards for the set-up and organisation of instrument tables. In addition, we encourage those responsible for developing and improving clinical procedures and checklists at hospitals to implement the relevant recommendations we have identified in the literature review.
We also recommend that master’s students in operating room nursing should draw up clinical procedures on the set-up and organisation of instrument tables for various surgical procedures, for inclusion in their thesis.
We further recommend that guidelines drawn up in other countries, which are not included in this literature review, be examined. Research on experiences and practices in connection with the set-up and organisation of instrument tables should be prioritised by operating room nurses in educational institutions and clinical settings.
Resistance to the use of standards in surgical departments may seem incomprehensible when the research community agrees that standardisation improves safety in the operating room. We call for a professional discussion with the spotlight on the use of standards in surgical departments.
The first author has participated in a programme for qualification as an associate professor and wishes to acknowledge and thank Emeritus Professor Ole Petter Rekvig for his professional guidance in connection with the work on this article.
The first author would also like to thank Inga Segtnan, Tove J. Berntsen, Ulla M. Svensson, Anna K. Roer and Anita Eilertsen for their useful input. Between them they have 170 years of experience as operating room nurses.
References
1. Reason J. Safety in the operating theatre – Part 2: Human error and organisational failure. Qual Saf Health Care. 2005;14(1):56–60.
2. Verdens helseorganisasjon (WHO). WHO guidelines for safe surgery 2009: safe surgery saves lives. Genève: WHO; 2009. Available at: https://apps.who.int/iris/bitstream/handle/10665/44185/9789241598552_eng.pdf (downloaded 11.11.2017).
3. Igesund U, Eide PH. Oppdekking av instrumenter på assistanse- og instrumentbord. In: Dåvøy GM, Eide PH, Hansen I, ed. Operasjonssykepleie. 2nd ed. Oslo: Gyldendal Akademisk; 2018. pp. 375–9.
4. Igesund U, Overvåg G, Rasmussen G, Rekvig OP. Kartlegging av prosedyrer for oppdekking av instrumentbord ved kirurgiske inngrep. Sykepleien Forskning. 2019;14(78413):(e-78413). DOI: 10.4220/Sykepleienf.2019.78413
5. Copeland A. Retained surgical sponge and other retained surgical items: Prevention and management. UpToDate; 2019. Available at: https://www.uptodate.com/contents/retained-surgical-sponge-gossypiboma-and-other-retained-surgical-items-prevention-and-management (downloaded 06.01.2020).
6. Wahr JA. Operating room hazards and approaches to improve patient safety. UpToDate; 2019. Available at: https://www.uptodate.com/contents/safety-in-the-operating-room? (downloaded 06.01.2020).
7. Verdens helseorganisasjon (WHO). Global guidelines for the prevention of surgical site infection. Genève: WHO; 2018. Available at: https://www.who.int/infection-prevention/publications/ssi-prevention-guidelines/en/ (downloaded 06.01.2020).
8. Nanji K. Prevention of perioperative medication errors. UpToDate; 2019. Available at: https://www.uptodate.com/contents/prevention-of-perioperative-medication-errors? (downloaded 06.01.2020).
9. Gould J. Overview of electrosurgery. UpToDate; 2019. Available at: https://www.uptodate.com/contents/overview-of-electrosurgery? (downloaded 06.01.2020).
10. Cowles CE. Fire safety in the operating room. UpToDate; 2019. Available at: https://www.uptodate.com/contents/fire-safety-in-the-operating-room? (downloaded 06.01.2020).
11. Weber DJ. Prevention of hepatitis B virus and hepatitis C virus infection among health care providers. UpToDate; 2020. Available at: https://www.uptodate.com/contents/prevention-of-hepatitis-b-virus-and-hepatitis-c-virus-infection-among-health-care-providers? (downloaded 01.03.2020).
12. Tan E, Russell K. Surgical plume and its implications: a review of the risk and barriers to a safe work place. ACORN: Journal of Perioperative Nursing in Australia. 2017;30(4):33–9.
13. Folkehelseinstituttet. Kunnskapsbasert praksis. Helsebiblioteket.no. Available at: https://www.helsebiblioteket.no/kunnskapsbasert-praksis (downloaded 28.01.2020).
14. Hjelen W, Sagbakken M. Operasjonssykepleiere mangler tid og kompetanse til å arbeide kunnskapsbasert. Sykepleien Forskning. 2018;13(69422):e-69422. DOI: 10.4220/Sykepleienf.2018.69422
15. Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Method. 2005;8(1):19–32.
16. Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5(1):69.
17. Peters M, Godfrey C, McInerney P, Soares C, Khalil H, Parker D. Methodology for JBI Scoping Reviews. I: Joanna Briggs Institute Reviewers’ Manual: 2015 edition/supplement. Adelaide, Australia: Joanna Briggs Institute; 2015. s. 1–24.
18. Khalil H, Peters M, Godfrey CM, McInerney P, Soares CB, Parker D. An evidence‐based approach to scoping reviews. Worldviews Evid Based Nurs. 2016;13(2):118–23.
19. Aveyard H. Doing a literature review in health and social care: a practical guide. 4th ed. London: Open University Press / McGraw- Hill Education; 2019.
20. Conner RL. Guidelines for perioperative practice: 2018 edition. Denver: AORN; 2018.
21. Association of Surgical Technologists, AST. About AST: AST guidelines. Available at: https://www.ast.org/AboutUs/Surgical_Technologists_Responsibilities/ (downloaded 16.03.2021).
22. Riksföreningen för operationssjukvård. Riksföreningen anser och rekommenderar. Available at: http://www.rfop.se/nationellt/riksfoereningen-anser-och-rekommenderar/ (downloaded 16.03.2021).
23. Verdens helseorganisasjon (WHO). WHO guidelines approved by the Guidelines Review Committee. Available at: https://www.who.int/publications/guidelines/en/ (downloaded 16.03.2021).
24. EORNA – European Operating Room Nurses Association. EORNA recommendations. Available at: https://eorna.eu/eorna-recommendations/ (downloaded 16.03.2021).
25. Folkehelseinstituttet. Kildevalg: kunnskapspyramiden med eksempler på kilder. Helsebiblioteket.no. Available at: https://www.helsebiblioteket.no/kunnskapsbasert-praksis/litteratursok/kildevalg (downloaded 16.03.2021).
26. Burlingame BL, Conner RL. Guideline for safe use of energy-generating devices. I : Conner RL, ed. Guidelines for perioperative practice: 2018 edition. Denver: AORN; 2018. pp. 129–55.
27. Fearon MC, Spruce L, Conner RL, Wood A. Guideline for team communication. In: Conner RL, ed. Guidelines for perioperative practice: 2018 edition. Denver: AORN; 2018. pp. 745–71.
28. Katsevman GA, Braca JA, Sedney CL, Hatchett L. Needlestick injuries among healthcare professionals in training: using the surgical ‘timeout’ and hand-off protocols to deter high-risk needlesticks. J Hosp Infect. 2017;95(1):103–4.
29. Van Wicklin SA, Conner RL. Guideline for sterile technique. In: Conner RL, ed. Guidelines for perioperative practice: 2018 edition. Denver: AORN; 2018. pp. 75–103.
30. Association of Surgical Technologists Education and Professional Standards Committee. Guidelines for best practices for establishing the sterile field in the operating room. AST; 2018. Available at: https://www.ast.org/AboutUs/Aseptic_Technique / (downloaded 06.01.2020).
31. Association of Surgical Technologists Education and Professional Standards Committee. Guidelines for best practices in bowel technique. AST; 2017. Available at: https://www.ast.org/uploadedFiles/Main_Site/Content/About_Us/Standard_Bowel_Technique.pdf (downloaded 06.01.2020).
32. Spruce L, Conner RL, Retzlaff CJ. Guideline for prevention of transmissible infections. In: Conner RL, ed. Guidelines for perioperative practice: 2018 edition. Denver: AORN; 2018. s. 499–531.
33. Association of Surgical Technologists Education and Professional Standards Committee. Guidelines for best practices for breaking down the sterile field. AST; 2018. Available at: https://www.ast.org/AboutUs/Aseptic_Technique/ (downloaded 06.01.2020).
34. Association of Surgical Technologists Education and Professional Standards Committee. Guidelines for best practices for sharps safety and use of the neutral zone. AST; 2017. Available at: https://www.ast.org/AboutUs/Surgical_Technologists_Responsibilities/ (downloaded 06.01.2020).
35. Ogg MJ, Conner RL. Guideline for sharps safety. In: Conner RL, ed. Guidelines for perioperative practice: 2018 edition. Denver: AORN; 2018. pp. 415–38.
36. DeGirolamo KM, Courtemanche DJ, Hill WD, Kennedy A, Skarsgard ED. Use of safety scalpels and other safety practices to reduce sharps injury in the operating room: what is the evidence? Can J Surg. 2013;56(4):263–9.
37. European Operating Room Nurses Association. Recommendations on prevention of retained surgical items. EORNA; 2019. Available at: https://eorna.eu/eorna-recommendations/ (downloaded 20.01.2020).
38. Wood A, Conner RL. Guideline for prevention of retained surgical items. In: Conner RL, ed. Guidelines for perioperative practice: 2018 edition. Denver: AORN; 2018. pp. 367–412.
39. Freitas PS, Silveira RCdCP, Clark AM, Galvão CM. Surgical count process for prevention of retained surgical items: an integrative review. J Clin Nurs. 2016;25(13/14):1835–47.
40. Gibbs VC. The prevention of retained surgical items: multi-stakeholder policy job aid-reference manual. San Francisco: NoThing Left Behind; 2018. Available at: http://nothingleftbehind.org (downloaded 06.01.2020).
41. Tande M, Tiberg E, Mykkeltveit IH, Danielsen LA, Helleland I, Christiansen MH. Kirurgisk telling: anbefalte rutiner for telling av utstyr under kirurgi. Helsebiblioteket; 2018. Available at: https://www.helsebiblioteket.no/fagprosedyrer/ferdige/kiriurgisk-telling-anbefalte-rutiner-for-telling-av-utstyr-under-kirurgi#preparation (downloaded 02.11.2018).
42. Burlingame BL, Conner RL. Guideline for medication safety. In: Conner RL, ed. Guidelines for perioperative practice: 2018 edition. Denver: AORN; 2018. pp. 295–327.
43. Riksföreningen för operationssjukvård. Riksföreningen anser och rekommenderar angående säker läkemedelshantering intraoperativt. Riksföreningen för operationssjukvård; 2018. Available at: http://www.rfop.se/nationellt/riksfoereningen-anser-och-rekommenderar/ (downloaded 20.01.2020).
44. Association of Surgical Technologists Education and Professional Standards Committee. Guidelines for safe medication practices in the perioperative area. AST; 2017. Available at: https://www.ast.org/AboutUs/Surgical_Technologists_Responsibilities/ (downloaded 06.01.2020).
45. Hauk L. Avoiding errors when preparing medications in the perioperative setting. AORN J. 2018;107(3):9–11.
46. Boytim J, Ulrich B. Factors contributing to perioperative medication errors: a systematic literature review. AORN J. 2018;107(1):91–107.
47. HOSPEEM, EPSU. Framework Agreement on prevention from sharp injuries in the hospital and healthcare sector. Brussel: EPSU; 2010. Available at: https://www.epsu.org/article/framework-agreement-prevention-sharp-injur… (downloaded 20.01.2020).
48. Folkehelseinstituttet (FHI). Smittevernveilederen: stikkuhell på sprøyter og andre blodeksponeringer: veileder for helsepersonell. Oslo: FHI; 2010 [oppdatert 15.01.2019, sitert 27.01.2020]. Available at: https://www.fhi.no/nettpub/smittevernveilederen/
49. Forskrift 6. desember nr. 1357 om utførelse av arbeid, bruk av arbeidsutstyr og tilhørende tekniske krav (forskrift om utførelse av arbeid). Available at: https://lovdata.no/dokument/SF/forskrift/2011-12-06-1357 (downloaded 04.05.2020).
50. Helse- og omsorgsdepartementet. Nasjonal helse- og sykehusplan 2020–2023. Available at: https://www.regjeringen.no (downloaded 04.05.2020).
51. Høyland S, Haugen AS, Thomassen Ø. Perceptions of time spent on safety tasks in surgical operations: a focus group study. Saf Sci. 2014;70:70–9.
52. Hemingway MW, O'Malley C, Silvestri S. Safety culture and care: a program to prevent surgical errors. AORN J. 2015;101(4):404–15.
53. Igesund U. Studenters deltakelse i kunnskapsbasert fagutvikling: pilotprosjekt i steril-assistanse ved videreutdanning i operasjonssykepleie. Nord tidsskr helseforsk. 2016;12(1):115–28.









Comments