Few patients receiving multimodal antiemetic treatment experience postoperative nausea after ambulatory surgery
Patients undergoing gynaecological laparoscopies and anorectal procedures suffered the most nausea, while those who underwent major breast cancer surgery experienced the least nausea.
Background: Ambulatory surgery, where the patient is operated on and discharged the same day, accounts for about 60 per cent of all elective surgery in Norway. Postoperative nausea is a known problem after anaesthesia and surgery, and one that can cause discomfort and, at worst, complications for the patient. In an ambulatory surgery setting, postoperative nausea can also lead to a prolonged stay in the postanaesthesia care unit or a need for unplanned hospital admission, which results in increased costs. International studies show that 37–57 per cent of patients report postoperative nausea after arriving home from ambulatory surgery.
Objective: To investigate what percentage of our ambulatory surgery patients experience postoperative nausea after discharge. We also wanted to investigate whether any patient groups are particularly vulnerable.
Method: The study is a cross-sectional study. Telephone follow-up the day after surgery was used to collect data using a structured questionnaire with set response options.
Results: A total of 2952 patients were included in the study and the response rate was 99 per cent. A general anaesthetic was administered to all patients in the form of total intravenous anaesthesia. Based on known risk factors, the majority of the sample had an increased risk of developing postoperative nausea. The study showed an incidence of postoperative nausea the day after the operation of 16 per cent, of which 14 per cent were slightly nauseous and only 2 per cent were very nauseous.
Conclusion: The study shows that the incidence of postoperative nausea after ambulatory surgery can probably be reduced through systematic, multimodal antiemetic prophylactic treatment.
Ambulatory surgery, where the patient is operated on and discharged the same day, currently accounts for about 60 per cent of all elective surgery in Norway. By using short acting anaesthetic agents and local anaesthesia, for example, to reduce pain, the patient is soon awake, mobile and ready to go home. This method is effective and saves costs by reducing the need for the hospitalisation of patients (1).
The clinical discharge criteria for a patient being able to go home after ambulatory surgery are that their circulatory and respiratory systems are stable, sufficient pain relief has been administered, they are not experiencing nausea and are mobile, and that they have had something to eat and drink and passed water (2).
Postoperative nausea
The exact pathophysiology of nausea and vomiting is complex, and many aspects remain unclear (3). The term ‘postoperative nausea and vomiting’ (PONV) is a generic term that includes nausea and/or vomiting following surgery (4, 5). In connection with ambulatory surgery, postoperative nausea can lead to prolonged stays in the department, hospitalisation (6) and a delay in the return to normal activity and work (7), which results in increased costs (8).
Nausea can also reduce patient satisfaction (8, 9). For ambulatory surgery patients, postoperative nausea may be particularly problematic since they do not have immediate access to specialised health care and intravenous antiemetic treatment after discharge (4). Nausea can cause problems with eating, drinking and taking medication. Vomiting can, at worst, lead to ruptures of the surgical wound, bleeding, aspiration of gastric contents and dehydration (6).
Postoperative nausea and vomiting after ambulatory surgery that occurs after discharge is known as postdischarge nausea and vomiting (PDNV), and even patients who did not initially experience nausea can suffer from this (7). Recent research has shown that patients experiencing postoperative nausea during their stay in the department are three times as likely to develop PDNV (4) and that the incidence of PDNV may be as high as 37–57 per cent after ambulatory surgery (4, 10, 11). In order to illustrate the extent of the number of patients exposed to PDNV, we refer to the USA, where approximately 35 million ambulatory surgery operations are performed every year (4).
Predisposing risk factors and prevention of postoperative nausea
Research has shown that being a woman (4, 5, 12) or a non-smoker (4, 5, 12), having a previous history of severe motion sickness or PONV (4, 5, 12) and postoperative use of opioids (4, 5, 12) are all predisposing risk factors to developing PONV.
Based on these factors, Apfel et al. devised the so-called Apfel score (12), which is an instrument used to measure the risk of PONV. The more risk factors that are present, the higher the risk of PONV. Patients with all four risk factors have an 80 per cent risk of developing PONV without preventive treatment (12). Use of inhalational anaesthesia or nitrous oxide and long-acting anaesthesia has also been shown to increase the risk of PONV (5).
In order to reduce the risk of PONV, Gan et al. recommend that the dosage of antiemetics is determined on a case-by-case basis according to estimated risk, in addition to providing adequate fluid therapy, minimising the use of postoperative opioids by administering other types of pain relief, as well as using regional anaesthesia or total intravenous anaesthesia (TIVA) instead of inhalational anaesthesia (13).
There has been discussion on whether special surgical procedures are associated with an increased risk of PONV (5, 13). The Consensus Guidelines for the Management of Postoperative Nausea and Vomiting (the SAMBA Guidelines) (13) describe an increased risk associated with laparoscopic procedures, gallbladder surgery and gynaecological surgery.
However, Apfel et al. (5) believe that it is the laparoscopic approach that is the determining factor, and not the type of procedure. Adequate pain relief appears to prevent postoperative nausea (14, 15), and a correlation has been shown between postoperative pain, opioid use and PDNV (10, 15).
In 2012, Apfel et al. presented a PDNV risk score (4) to calculate patients’ risk of developing PDNV with the following risk factors: PONV whilst in the postanaesthesia care unit (4, 10), female gender (4, 10), age < 50 years (4, 10), history of PONV (4, 10) and opioid administration in the postanaesthesia care unit (4, 10). Here too, the risk of PDNV increases with the number of risk factors. The Society for Ambulatory Anesthesia (SAMBA) recommends both of the risk-scoring tools devised by Apfel et al. (4, 12) in the SAMBA Guidelines (13).
Objective of the study
The objective of our study was to investigate what percentage of patients experienced PDNV following ambulatory surgery. We also wanted to investigate whether there were any disparities based on gender and type of surgery.
After the department implemented new guidelines for targeted, systematic, nausea prophylaxis internally, we wanted to investigate the incidence, severity and distribution of PDNV after discharge in a large and wide-ranging patient data set. The knowledge gained from the study may be important for further work on preventing and treating postoperative nausea after ambulatory surgery.
Method
The study was a cross-sectional study in which we collected data at the Oslo University Hospital between September 2011 and August 2015. The data were collected during a follow-up telephone call the day after surgery. We used a structured questionnaire (see the appendix), which we obtained from another ambulatory surgery department. The ambulatory surgery department at the Oslo University Hospital, where we conducted the study, introduced the questionnaire in 2011 as a quality improvement measure.
The questionnaire covers pain, nausea, bleeding, mobilisation, sleep, information and satisfaction. It consists of ten questions with set response options. In addition, it provides data on when the operation was performed, the type of surgery, the form of anaesthesia and the patient’s gender. We only used the data relating to nausea in this study. The question about postoperative nausea was as follows: ‘Have you felt nauseous since returning home?’ The patient assessed their own nausea based on the response options ‘No’, ‘A little’ or ‘A lot’.
Inclusion criteria for telephone follow-up
The inclusion criteria for following up a patient by telephone were that they were aged 18 or older and were discharged as planned after surgery or stayed overnight at a regular patient hotel that was not staffed with medical personnel. In addition, the patient had to be able to conduct the telephone conversation in Norwegian, Swedish, Danish or English.
Data collection
In this study, we chose only to consider patients who had received a general anaesthetic in the form of TIVA, since there were few patients who had received a different type of anaesthetic as the main form of sedation. Patients who met the inclusion criteria were given the questionnaire upon discharge from the ambulatory surgery department. They were informed that we would call them the next day to collect their responses. Where surgery had been performed on a Friday or the day before a public holiday, we called the patient the next working day.
During the telephone follow-up, a nurse marked the patient’s response on a hard copy of the questionnaire. An external specialist nurse then entered the data in a statistical program. Responses or data that were missing were coded as ‘missing’.
Seven dedicated postanaesthesia care nurses undertook the telephone follow-up. In order to safeguard the comparability of the data, all recorded data relate to the patient’s condition on the day after returning home from ambulatory surgery. The procedure required the nurses to follow the same questionnaire and ask the questions in the same way.
Primary nursing care was practised at the department. In order to ensure that the patients felt free to express their opinion, they were called by a different nurse to the one who was responsible for them on the day of surgery.
Anaesthesia, nausea prophylaxis and preventive pain relief
All patients in the study received propofol-based TIVA, which is known to result in rapid awakening. TIVA also reduces the risk of PONV during the first few hours after surgery, and is less likely to cause nausea than inhalational anaesthesia (16–19). Remifentanil was used as an opioid. The patients were ventilated with oxygen and air. Perioperatively, the opioid fentanyl was also administrated intravenously, in addition to local anaesthetic wound infiltration in order to prevent pain.
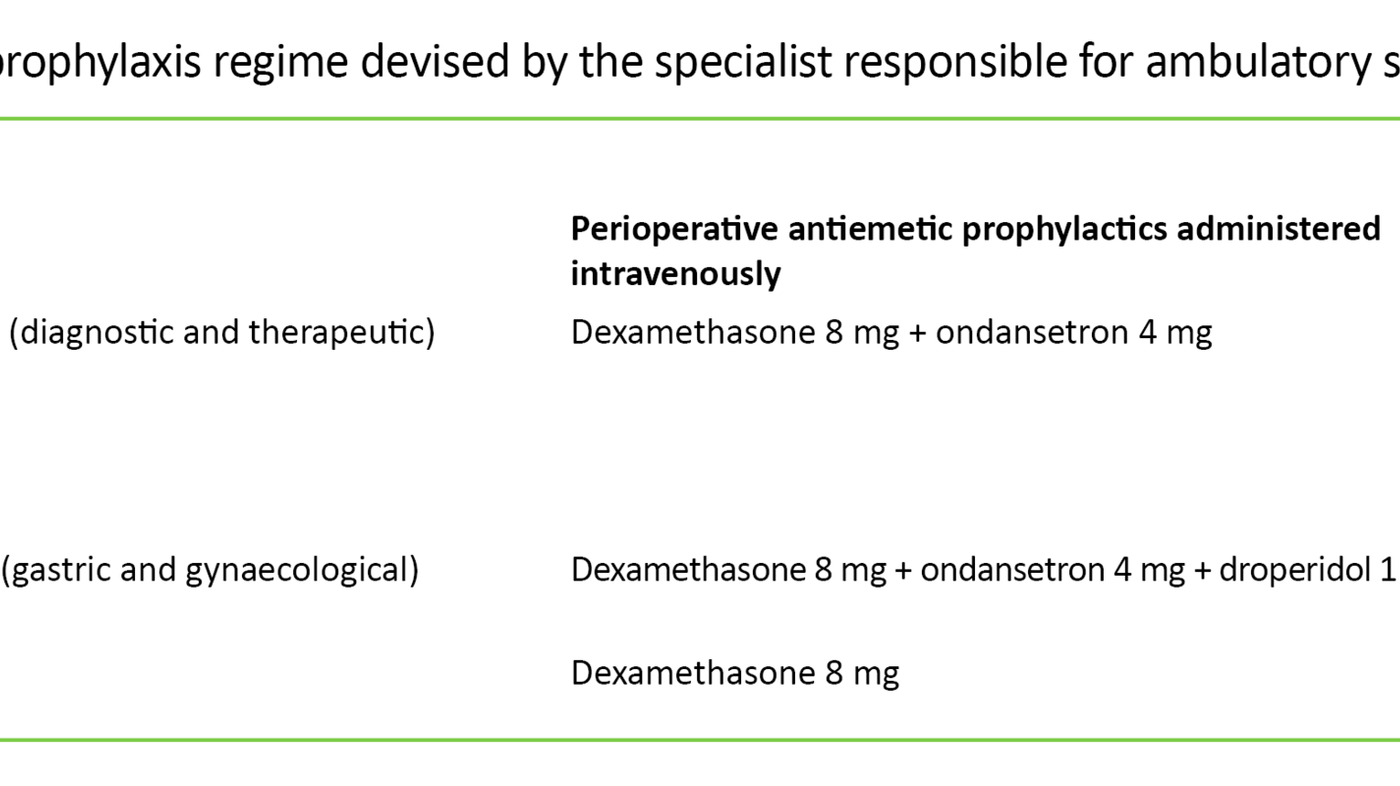
The patients were subject to perioperative antiemetic prophylaxis according to an internal standardised regime (Table 1) devised by the specialist responsible for ambulatory surgery in line with relevant knowledge and experience. The nausea prophylaxis consisted of three different drug combinations based on expected risk of nausea according to the type of surgical procedure. Some patient groups were also given a prescription for an oral opioid (codeine, tramadol), while others received tablets to take with them. If needed, patients were also given a potent opioid (oxycodone) to take home after surgery, which was to be taken for 1–3 days.
Premedication in the form of a combination of paracetamol and a non-steroidal anti-inflammatory drug (NSAID) or a COX-2 inhibitor was given as pain prophylaxis where there were no contraindications.
Postoperative pain was further treated with fentanyl intravenously, paracetamol and NSAID or COX-2 inhibitors as well as oral opioids if needed. The patients were given a prescription for paracetamol and NSAID or COX-2 inhibitors upon discharge.
Ethical considerations
A nurse informed the patients that answering the questionnaire was voluntary, that the responses were anonymous and that the objective of the survey was internal quality improvement. By answering the questionnaire, the patient was regarded as consenting to participation in the survey. In order to comply with the duty of confidentiality, the patient was contacted on their own mobile phone.
The Data Protection Officer at Oslo University Hospital defined the survey as a quality improvement initiative and, since all data were anonymous, did not consider it to be subject to the obligation to give notification. The head of the ambulatory surgery department granted permission to publish the data. The study did not entail any kind of additional interventions.
Statistical analysis
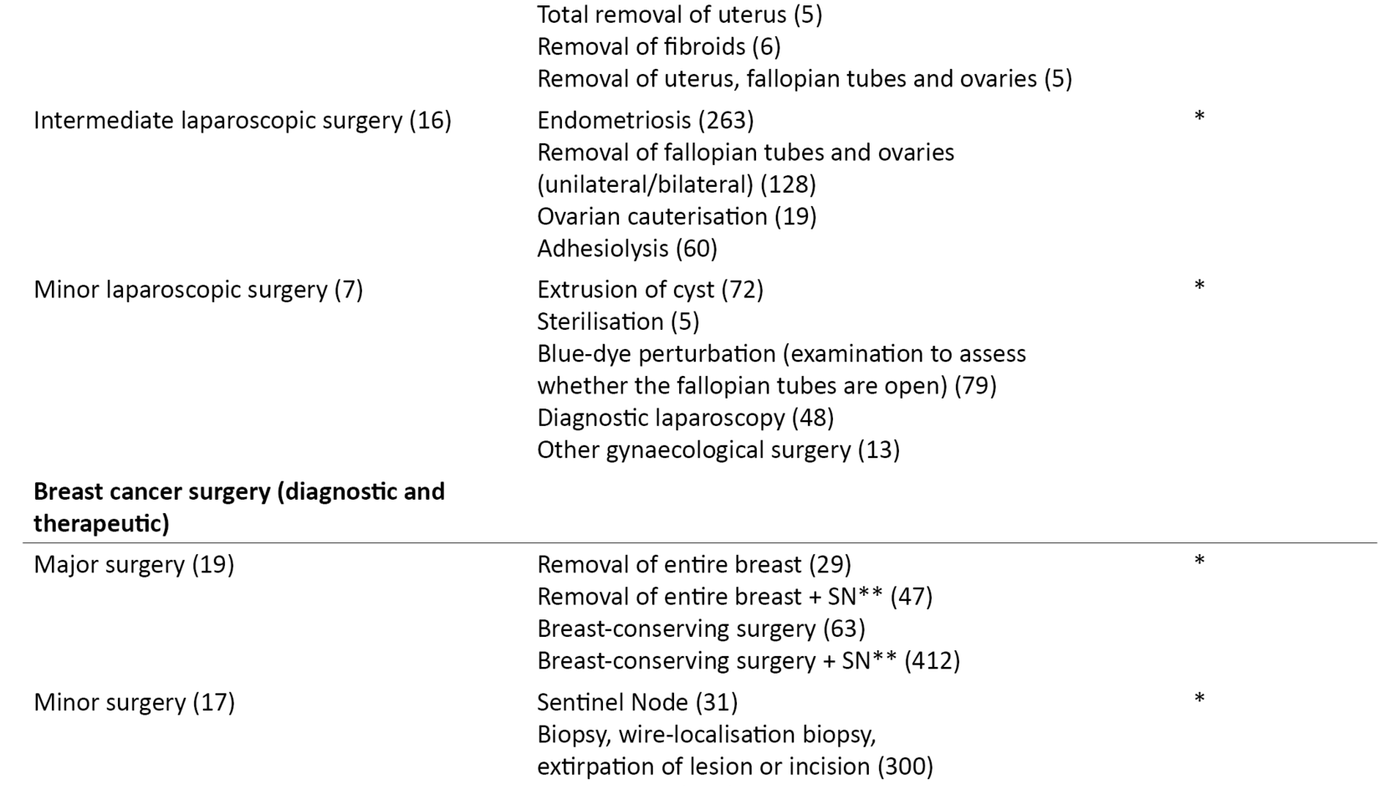
We divided the patient categories into subgroups by type of surgery, magnitude of the operation and expected risk of PONV (Table 2). The breakdown was carried out in consultation with the specialist responsible for ambulatory surgery at the hospital. In the bivariate analysis, we chose to dichotomise the response options ‘No’, ‘A little’ and ‘A lot’ to ‘Not nauseous’ and ‘Nauseous’ (slightly and very) as we considered the occurrence or absence of nausea to be most clinically relevant to this study.
We performed statistical analyses using IBM SPSS Statistics, version 22.0 (SPSS Inc., Chicago, IL, USA). We performed frequency analyses to describe the sample, and bivariate analyses with chi-square tests to describe the incidence of PDNV within the various explanatory variables. In the analysis of the results shown in Table 3, we used McNemar’s test to indicate significance. The significance level was set at p < 0.05.
Results
Out of the 3204 patients who were followed up in a phone call, 2952 were included in the study. The response rate was 99 per cent, and the data set contained < 5.5 per cent missing data. Women accounted for 79 per cent of the patients included, 40 per cent underwent gastric surgery, 24 per cent had a gynaecological procedure and 36 per cent had breast cancer surgery (diagnostic and therapeutic).
Laparoscopic abdominal procedures accounted for a total of 46 per cent of all surgical procedures. The surgical method, type of operation and gender distribution are shown in Table 2. The anaesthesia may have been converted to inhalational anaesthesia in a few cases without this being recorded since inhalational anaesthesia was not a response option in the questionnaire.
Nausea after returning home and surgical procedures
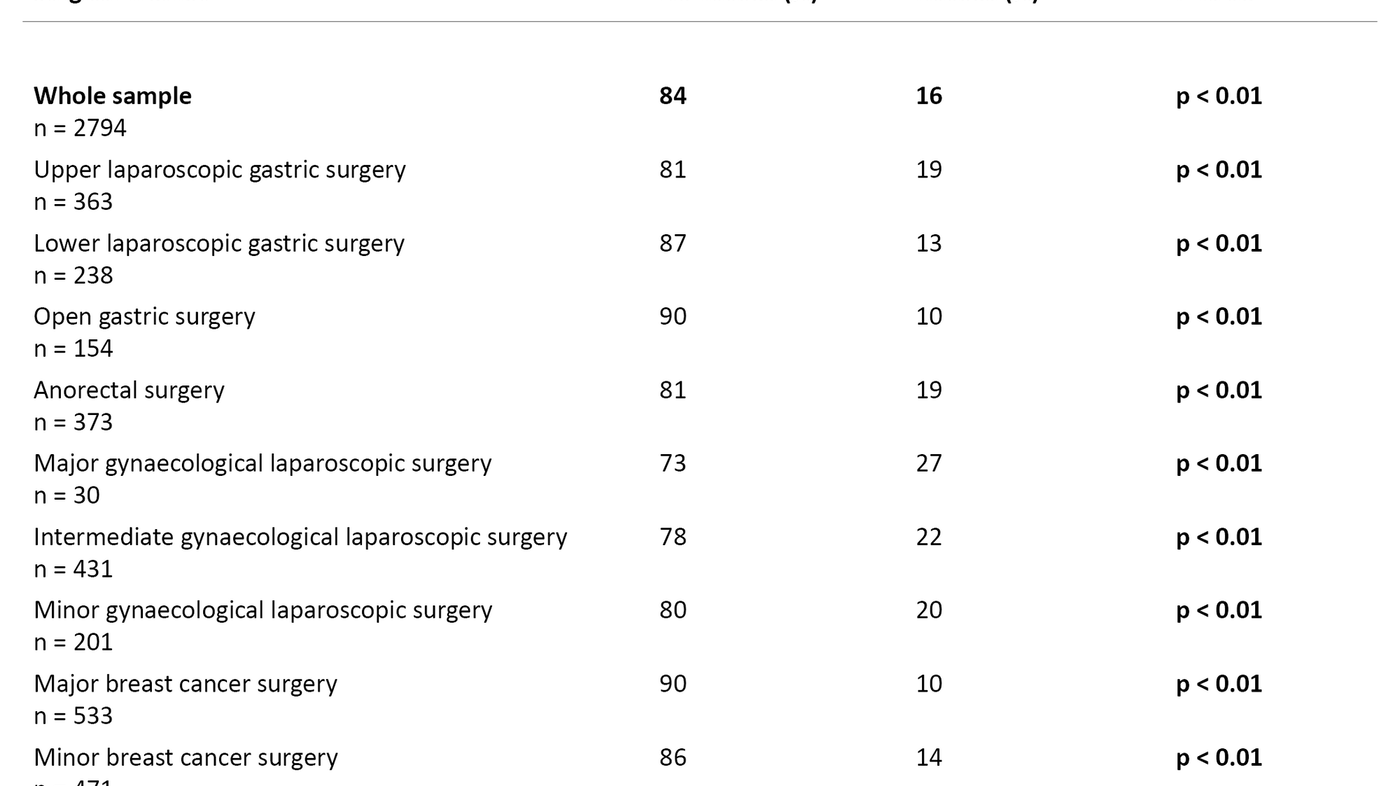
In total, 16 per cent of the ambulatory surgery patients experienced PDNV after returning home. Of these, 14 per cent reported that they were slightly nauseous after returning home, and 2 per cent were very nauseous. In terms of the gender breakdown, 17 per cent of the women and 12 per cent of the men reported experiencing PDNV (p < 0.05).
Patients undergoing a gynaecological laparoscopy were most prone to PDNV (20–27 per cent) (p < 0.01), followed by upper laparoscopic gastric surgery (19 per cent) (p < 0.01) and anorectal surgery (19 per cent) (p < 0.01). The lowest incidence of PDNV was reported by those who had undergone major breast cancer surgery (10 per cent) (p < 0.01) (Table 3).
Discussion
Comparing the incidence of PDNV across studies is a challenge due to differences in patient samples, methods of anaesthesia, operation techniques, types of operation and in when the PDNV was measured. Comparisons have also become more complicated due to the major advancements in the fields of anaesthesia and surgery over the past decade.
This study showed a total incidence of PDNV of 16 per cent the day after surgery. The sample had an increased risk of PDNV given that it was made up of almost 80 per cent women and nearly half of the operations consisted of laparoscopic abdominal procedures, which are associated with a greater risk of postoperative nausea.
Apfel et al. (4) reported a 37 per cent incidence of PDNV in the first 48 hours after surgery in a multi-centre study of 2170 ambulatory surgery patients. The patients underwent various surgical procedures. The majority received prophylactic antiemetics, and women accounted for a lower percentage than in our study.
In the study by Apfel et al. (4), inhalational anaesthetics were administered to all of the patients, which may have contributed to the higher incidence of PDNV. In our study, all patients received TIVA.
Kappen et al. (11) examined 11 613 patients, all of whom received adequate nausea prophylaxis. The gender distribution was 50/50, and about half of the patients received inhalational anaesthesia. According to Kappen et al. (11), the sample was not a distinctly high-risk population, and consisted only of elective patients, some of whom underwent ambulatory surgery. Nevertheless, 41–43 per cent of the patients experienced nausea within the first 24 hours after surgery, which was considered to be an unexpectedly high incidence of PDNV.
The relatively low incidence of PDNV in our study may be the result of all patients receiving multimodal antiemetic prophylactic treatment with both propofol-based TIVA and antiemetics according to a standardised regime, with medications that are recommended in the SAMBA Guidelines (13). Individual risk scoring was not practised in the department, so some patients probably received more antiemetics than they should have based on the number of risk factors, while some high-risk patients probably did not receive enough.
Gynaecological patients had highest incidence of PDNV
As expected, the women in our study reported a higher incidence of PDNV than the men, which concurs with earlier research (4, 5, 10, 12). In terms of procedures on the women, the gynaecological patients reported the highest incidence of PDNV (20–27 per cent) according to the data, while the breast cancer patients had the lowest incidence of PDNV (10–14 per cent). Chen et al. (20) report a 14 per cent incidence of PDNV after breast surgery performed using TIVA, which is in line with our findings.
A contributing factor to the high incidence of PDNV among the gynaecological patients in our study may be the surgical method (5, 13). Paech et al. (21) presented a similar result. They reported a 25 per cent incidence of nausea following a gynaecological laparoscopy using TIVA combined with analgetic and antiemetic prophylaxis.
Use of a multimodal antiemetic prophylaxis was also demonstrated by Scruderi et al. (22). Using a multimodal antiemetic approach similar to ours, 12 per cent of the patients reported PDNV after a gynaecological laparoscopy. In the same study, 32 per cent of the patients who received inhalational anaesthesia without antiemetics experienced PDNV. The patients who received multimodal treatment could also be discharged sooner than the other patients in the study (22).
The patients who underwent upper laparoscopic gastric surgery reported the second highest incidence of nausea in our study, with 19 per cent experiencing PDNV. The majority were women, and cholecystectomies accounted for 98 per cent of the procedures, which is a predictor of an increased risk of postoperative nausea.
In another study (23) where a multimodal approach including TIVA was used and most of the patients were women, the incidence of PDNV after a laparoscopic cholecystectomy was 20 per cent, which is in accordance with our findings.
Less nausea following lower laparoscopic gastric surgery
The two gastric surgery patient groups undergoing a lower laparoscopy and open surgery showed a lower incidence of nausea than the other gastric surgery patients in the study. Despite a laparoscopic operating method being used, the patients who underwent a lower laparoscopy reported a lower incidence of nausea than those who had undergone minor breast operations.
One explanation may be that 99 per cent of the lower laparoscopic procedures consisted of surgery for inguinal hernia, where the latest technique entails operating outside the peritoneum using the so-called total extraperitoneal (TEP) approach. TEP has been shown to be a gentler surgical technique (24) that may reduce the risk of nausea in comparison with the other laparoscopic procedures in the study in which surgery takes place within the peritoneum. In the group with open gastric surgery, minor operations were generally performed.
High incidence of PDNV after anorectal surgery
The high incidence of PDNV after anorectal surgery was unexpected, as these operations are considered to be relatively simple and uncomplicated. In addition, the majority of the patients were men. We had expected to see a higher incidence of PDNV among the breast cancer patients – who were women that had undergone relatively major operations – than among those who had had anorectal surgery.
Although the anorectal group received dexamethasone as an antiemetic prophylactic, which has proven to be effective (25), the incidence of PDNV was the same as for the group who underwent upper laparoscopic gastric surgery. It is difficult to know the reason for the high incidence of PDNV, but postoperative pain is a known problem after anorectal surgery (26), which can cause nausea (15). Many of the patients also received a prescription for an oral opioid, which may also have contributed to nausea.
By comparison, Coloma et al. (25) found a PDNV incidence of 8 per cent after anorectal surgery, where dexamethasone was given as an antiemetic prophylactic and the surgery was performed under local anaesthesia and sedation. In our study, all patients received a general anaesthetic in the form of TIVA. This may indicate more extensive surgery in our patients and a need to increase nausea prophylaxis for this patient group.
PDNV and postoperative pain
The incidence of PDNV seems to remain relatively stable despite new knowledge. One of the reasons may be that advancements in the fields of surgery and anaesthesia have made it possible to carry out more extensive and more complicated ambulatory surgery operations. This in turn may affect both the incidence of pain and the need for postoperative opioids (27).
Thagaard et al. (14) point out that non-opioid pain relief is important in preventing postoperative nausea. Using ketorolac perioperatively, they showed a reduction in postoperative pain, a reduced need for opioids and a lower incidence of postoperative nausea.
Odom-Forren et al. (10) examined the incidence of PDNV during the first week after surgery in 248 ambulatory surgery patients. They found a total PDNV incidence of 57 per cent, which was considerably higher than expected. Six per cent of the patients were still experiencing PDNV seven days after the operation (10). Odom-Forren et al. found a correlation between PDNV and postoperative pain, with patients with a high pain score reporting a higher incidence of PDNV (10, 15), and the likely cause being a higher opioid consumption (10).
Strengths and limitations of the study
The study’s strength is the large sample within three patient groups and the high response rate. In addition, all patients had been subject to the same type of anaesthesia as well as pain and nausea prophylaxis under a standardised regime.
A limitation of the study is that the questionnaire we used contained little background information on the patients. In future studies it will be important to include more information about the patients, such as age and known risk factors for developing PONV or PDNV. The data for postoperative pain were incomplete, which prevented us from investigating the link between nausea and pain, and therefore reduced the basis of interpretation. Furthermore, the incidence of vomiting should be recorded in connection with information about PONV or PDNV, as the terms include both nausea and vomiting.
Conclusion
In this study, the majority of the sample had an increased risk of developing PDNV. Nevertheless, a relatively low percentage experienced PDNV compared to other recent studies. These findings may imply that a systematic, multimodal approach to antiemetic prophylaxis can contribute to a low incidence of postoperative nausea after ambulatory surgery.
The women in the study reported a higher incidence of nausea than the men. A breakdown by type of procedure showed that patients undergoing laparoscopic surgery experienced the most nausea, particularly after a gynaecological laparoscopy, while those who had undergone major breast cancer surgery were least nauseous. Anorectal surgery patients reported an unexpectedly high incidence of nausea, which is something that should be followed up further.
In order to prevent and reduce the incidence of both PONV and PDNV, the incidence should be documented according to risk factors and prophylaxis throughout the entire course of the patient’s clinical pathway.
References
1. Ræder J, Nordentoft J. Dagkirurgi og anestesi. Tidsskrift for Den norske legeforening. 2010;130(7):742–6.
2. Norsk anestesiologisk forening. Norsk standard for anestesi [Internet]. Oslo: Norsk anestesiologisk forening, ALNSF; 2010 [cited 06.05.2016]. Available at: https://www.alnsf.no/alnsf/norsk-standard-for-anestesi.
3. Blackburn J, Spencer R. Postoperative nausea and vomiting. Anaesthesia & Intensive Care Medicine. 2015;16(9):452–6.
4. Apfel CC, Philip BK, Cakmakkaya OS, Shilling A, Shi Y-Y, Leslie JB, et al. Who is at risk for postdischarge nausea and vomiting after ambulatory surgery? Survey of Anesthesiology. 2013;57(1):1.
5. Apfel CC, Heidrich FM, Jukar-Rao S, Jalota L, Hornuss C, Whelan RP, et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth. 2012b;109(5):742–53.
6. Keyes M. Management of postoperative nausea and vomiting in ambulatory surgery: The big little problem. Clin Plast Surg. 2013;40(3):447–52.
7. Carroll NV, Miederhoff P, Cox FM, Hirsch JD. Postoperative nausea and vomiting after discharge from outpatient surgery centers. Anesth Analg. 1995;80(5):903–9.
8. Parra-Sanchez I, Abdallah R, You J, Fu AZ, Grady M, Cummings K 3rd, et al. A time-motion economic analysis of postoperative nausea and vomiting in ambulatory surgery. Can J Anaesth. 2012;59(4):366–75.
9. Odom-Forren J, Hooper V, Moser DK, Hall LA, Lennie TA, Holtman J, et al. Postdischarge nausea and vomiting: management strategies and outcomes over 7 days. J Perianesth Nurs. 2014;29(4):275–84.
10. Odom-Forren J, Jalota L, Moser DK, Lennie TA, Hall LA, Holtman J, et al. Incidence and predictors of postdischarge nausea and vomiting in a 7-day population. J Clin Anesth. 2013;25(7):551–9.
11. Kappen HT, Moons GMK, Van Wolfswinkel JL, Kalkman AC, Vergouwe AY, Van Klei AW. Impact of risk assessments on prophylactic antiemetic prescription and the incidence of postoperative nausea and vomiting: A cluster-randomized trial. Anesthesiology. 2014;120(2):343–54.
12. Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91(3):693.
13. Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, Meyer TA, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118(1):85–113.
14. Thagaard KS, Jensen HH, Raeder J. Analgesic and antiemetic effect of ketorolac vs. betamethasone or dexamethasone after ambulatory surgery. Acta Anaesthesiol Scand. 2007;51(3):271–7.
15. Odom-Forren J, Rayens MK, Gokun Y, Jalota LM, Radke O, Hooper V, et al. The relationship of pain and nausea in postoperative patients for 1 week after ambulatory surgery. Clin J Pain. 2015;31(10):845–51.
16. Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. The New England Journal of Medicine. 2004;350 (24):2441–51.
17. Yoo YC, Bai SJ, Lee KY, Shin S, Choi EK, Lee JW. Total intravenous anesthesia with propofol reduces postoperative nausea and vomiting in patients undergoing robot-assisted laparoscopic radical prostatectomy: a prospective randomized trial. Yonsei Med J. 2012;53(6):1197–202.
18. Eikaas H, Raeder J. Total intravenous anaesthesia techniques for ambulatory surgery. Curr Opin Anaesthesiol. 2009;22(6):725–9.
19. Kumar G, Stendall C, Mistry R, Gurusamy K, Walker D. A comparison of total intravenous anaesthesia using propofol with sevoflurane or desflurane in ambulatory surgery: systematic review and meta-analysis. Anaesthesia. 2014;69(10):1138–50.
20. Chen HP, Hsu YH, Hua KC, Lin CC, Lo YF, Yu HP. Comparison of sevoflurane versus propofol under auditory evoked potential monitoring in female patients undergoing breast surgery. Biomedical Journal. 2013;36(3):125–31.
21. Paech MJ, Rucklidge MWM, Lain J, Dodd PH, Bennett E-J, Doherty DA. Ondansetron and dexamethasone dose combinations for prophylaxis against postoperative nausea and vomiting. Anesth Analg. 2007;104(4):808–14.
22. Scuderi PE, James RL, Harris L, Mims GR 3rd. Multimodal antiemetic management prevents early postoperative vomiting after outpatient laparoscopy. Anesth Analg. 2000;91(6):1408–14.
23. Habib AS, White WD, Eubanks S, Pappas TN, Gan TJ. A randomized comparison of a multimodal management strategy versus combination antiemetics for the prevention of postoperative nausea and vomiting. Anesth Analg. 2004;99(1):77–81.
24. Nawaz T, Ayub MW, Murad F, Ali Q, Khan A, Anwar I. Comparison of laparoscopic total extra peritoneal (TEP) techniques versus transabdominal preperitoneal (TAPP) technique for inguinal hernia repair. Journal of Rawalpindi Medical College (JRMC). 2015;19(3):220–2.
25. Coloma M, Duffy LL, White PF, Kendall Tongier W, Huber PJ Jr. Dexamethasone facilitates discharge after outpatient anorectal surgery. Anesth Analg. 2001;92(1):85–8.
26. Vinson-Bonnet B, Higuero T, Faucheron JL, Senejoux A, Pigot F, Siproudhis L. Ambulatory haemorrhoidal surgery: systematic literature review and qualitative analysis. Int J Colorectal Dis. 2015;30(4):437–45.
27. Rawal N. Postoperative pain treatment for ambulatory surgery. Best Practice & Research Clinical Anaesthesiology. 2007;21(1):129–48.









Comments