Palliative patients get greater relief from early screening of symptoms and implementation of measures
Fatigue, dry mouth and loss of appetite are the most distressing symptoms, according to a screening with the ESAS tool.
Background: Palliative cancer patients experience a complex clinical picture. Research shows little correlation between nurses’ impressions of the patient’s symptoms and the patient’s experience of their own symptoms. National guidelines recommend the use of screening tools to chart and evaluate the patient’s symptoms systematically.
Objective: The objective of the study is to elucidate palliative cancer patients’ self-reporting of symptoms on admission to and discharge from a palliative care unit.
Method: The study was conducted at the Palliative Care Unit, University Hospital North Norway, Harstad, in the period 2008–2016. The study uses data from the Edmonton Symptom Assessment System (ESAS). The dataset is presented using descriptive statistics.
Results: The study comprised 274 patients, of whom 135 were women and 139 men. Fatigue, dry mouth and loss of appetite emerge as the most distressing symptoms. Women exhibited a greater symptom burden than men, with the exception of shortness of breath. There was a significant reduction in all symptoms on discharge.
Conclusion: Good palliative care entails that nurses are attentive to and have knowledge of the patients’ symptoms. Systematic registration of symptom data facilitates the detection of symptoms that might otherwise have been overlooked. Early screening and treatment may reduce the risk of major, problematic symptoms over a lengthy period, and therefore give improved quality of life.
Palliative or supportive care is a new area of expertise in both the Norwegian and the international context. Cecily Saunders (1918–2005), who worked at St. Christopher’s Hospice in London (1), established modern palliation in the 1960s. Throughout the 1990s, palliation received more attention in Norway, partly as a result of the establishment of the Norwegian Association for Palliative Medicine (2).
In 2007, the Norwegian Directorate for Health and Social Affairs issued for the first time a national action programme with guidelines for palliation in cancer care (3). A new Official Norwegian Report on palliative care was issued in 2017: På liv og død (A matter of life or death) (1), which reviewed and revised existing palliative care programmes and set out parameters for future programmes.
Many patients in the palliative phase have both physical and mental problems, and often present a complex picture that can vary over time. Good knowledge of the symptoms of this patient group is a prerequisite for optimal care of the patient.
Earlier research
International research has shown that there is little correlation between nurses’ impressions of the patient’s symptoms and the patient’s actual clinical picture (4). Nurses’ knowledge of the patient’s clinical picture can be improved by using systematic screening tools, particularly if the information acquired from using such tools is included in nursing documentation (5).
There is a clear correlation between a high symptom score on the Edmonton Symptom Assessment System (ESAS) and the implementation of clinical actions (6). A number of international studies have presented statistical data from ESAS (6–9). One of the main goals of palliative care is to give higher quality of life through better control of symptoms.
Myhra (2010) points to a need to conduct further research on ESAS in which respondents are included, at all of Norway’s palliative care units (10). Earlier research has described different clinical pictures, depending on gender, for various forms of cancer at different stages. On the other hand, there is a lack of information on gender-specific differences in respect of palliative cancer patients (11, 12).
The national action programme for palliative cancer care (13) provides guidelines for treatment, competence and the organisation of palliative services in Norway. These guidelines formed the framework used when the palliative care unit at the University Hospital North Norway, Harstad (UNN Harstad) was established in 2008.
Objective of the study
The objective of the study was to identify:
- how palliative cancer patients report their symptoms on initial admission to the palliative care unit at UNN Harstad;
- whether male and female cancer patients report a different clinical picture; and
- how patients’ experience of symptoms changes during their initial stay at a palliative care unit.
Method
Palliative Care Unit - UNN Harstad
When the palliative care unit at UNN Harstad was established in 2008, it was the first palliative ward in the Northern Norway Regional Health Authority. The unit is open five days a week from Monday to Friday as a palliative centre with three to four beds and an outpatient clinic. It offers supervision and guidance to the primary health service as well as home visits. Over 90 per cent of the unit’s patients have a cancer diagnosis.
Design
The study is designed as a retrospective, quantitative study using anonymised data from the quality database at the Palliative Care Unit, UNN Harstad. The theoretical perspective is palliative nursing.
ESAS
The Edmonton Symptom Assessment System (ESAS) is a validated (8, 9, 14) and internationally recognised self-reporting tool for systematic registration of symptoms by patients receiving palliative care (15). The screening tool was devised in Canada (15) and is used both nationally and internationally.
Systematic use of ESAS is one of the quality measurements in palliative care (1, 2). The tool exists in several versions and has been translated to a number of languages. The Norwegian version uses a numerical scale from 0–10, where 0 represents ‘no symptoms’ and 10 represents ‘worst possible symptoms’.
ESAS is used to chart pain when resting, pain when active, fatigue, nausea, shortness of breath, dry mouth, appetite, feeling anxious or nervous, and sadness or depression. In addition, it includes a more general question: ‘Overall, how do you feel today?’ The ESAS form used in this study also charts constipation, in line with recommendations from earlier research (10, 15–17).
The patients’ symptoms are charted on a daily basis using ESAS. On first admission to the unit, the patient completes the form together with a nurse to avoid misunderstandings. Afterwards, if the patient so wishes, he/she can complete it on their own. The conversation between the patient and the nurse or doctor in this process is important and valuable, but is not discussed further in this article.
In 2010, a revised version of the form was launched: ESAS-R (18). In this study, we have used a traditional ESAS form.
Diagnosis
The International Statistical Classification of Diseases and Related Health Problems, ICD-10, was used to classify cancer diagnoses (19).
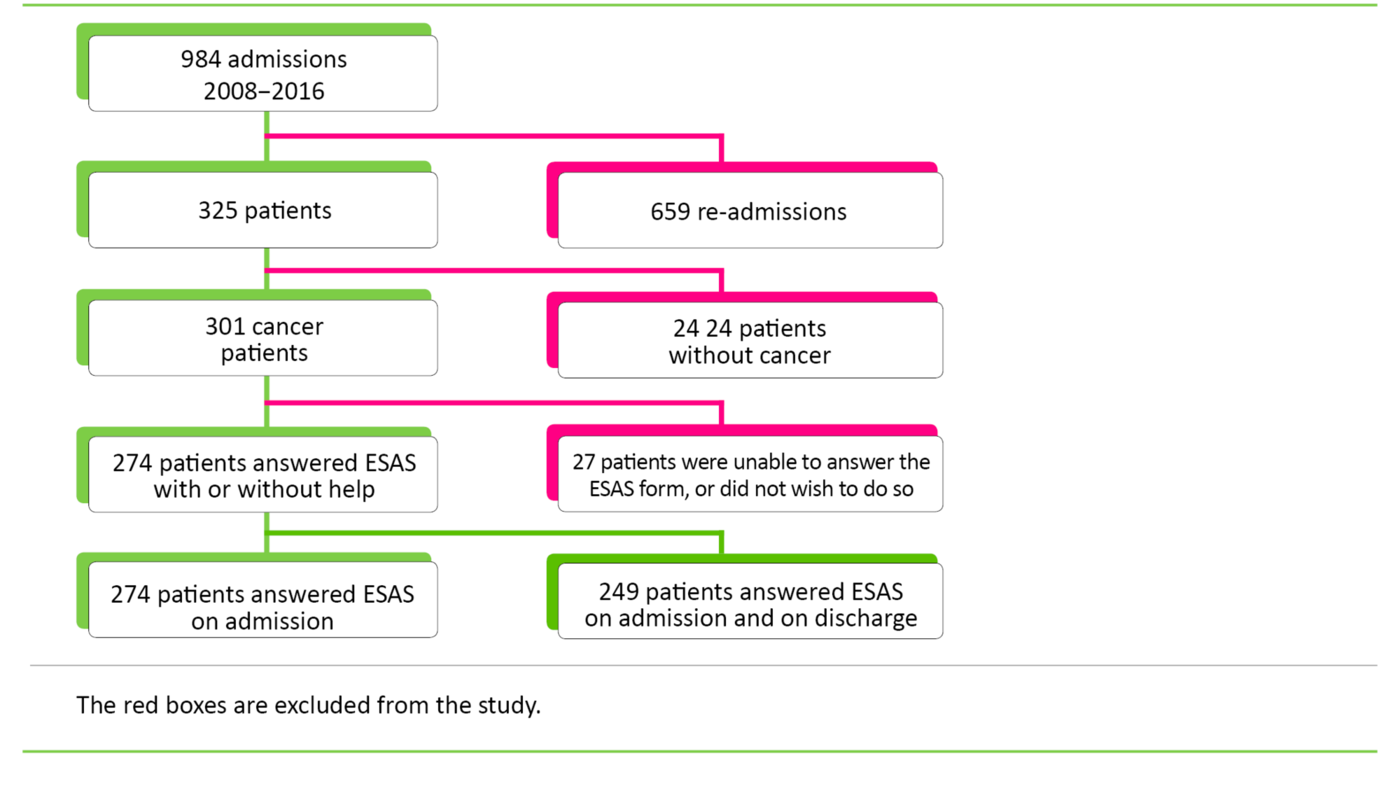
Inclusion
We employed the following inclusion criteria: 1) The patient must have a cancer diagnosis on initial admission, and 2) symptoms must be charted using ESAS.
Statistical analyses
Patient data are directly registered in IBM SPSS (Statistical Package for the Social Sciences). We used IBM SPSS version 22–23 and Microsoft Excel Version 2010 for the statistical analyses. Excel was used to select the week’s first and last symptom score as well as to prepare statistical figures. We used descriptive statistics to present the dataset. The Shapiro-Wilk and Kolmogorov-Smirnov tests demonstrated a skewed distribution.
Based on earlier research (6, 20, 21), we categorised the ESAS symptoms into four categories: ‘No symptoms’, ‘Mild symptoms’, ‘Moderate symptoms’, and ‘Pronounced symptoms’ (Figure 2). The Mann-Whitney U test was used to test differences between genders (Figure 3). We omitted gender-specific diagnoses such as breast cancer, gynaecological cancers and prostate cancer.
We used McNemar’s test to examine whether there were significant differences in the clinical picture between the first and last charting of symptoms (Figure 4). Only patients screened using ESAS both on admission and discharge were included in this specific analysis. Data were dichotomised, and the scores 0, 1 and 2 were compared against the scores 3–10. The goal is that the patient will score the symptom as 0–2.
In the case of pain when resting, the goal is defined as NRS (Numeric Rating Scale) ≤3 in the Standard for Palliative Care (2). We used the same categorisation as for the other symptoms. This corresponds with the goal that daily clinical practice attempts to achieve. The significance level was set at 0.05. We carried out a Bonferroni correction to reduce the risk of Type 1 errors.
Ethics
The material we used in this project consists of anonymised data from the quality database at the Palliative Care Unit, UNN Harstad. The study was subject to administrative processing by the Norwegian Social Science Data Services (NSD), now the Norwegian Centre for Research Data, via the data protection officer at UNN.
The Regional Committee for Medical and Health Research Ethics (REC North) has determined that the database is not subject to the Health Research Act and therefore does not require further approval (reference number 2015/1661/REC North). We collected all data as a routine part of patient treatment and the study has therefore not entailed any kind of extra strain on the informants.
To ensure full anonymity, we omitted the patients’ ID numbers when extracting data from the quality database. The sequence of observation units was randomised and the different diagnoses were combined in ten large groups, none of which had less than five patients (Table 1).
Results
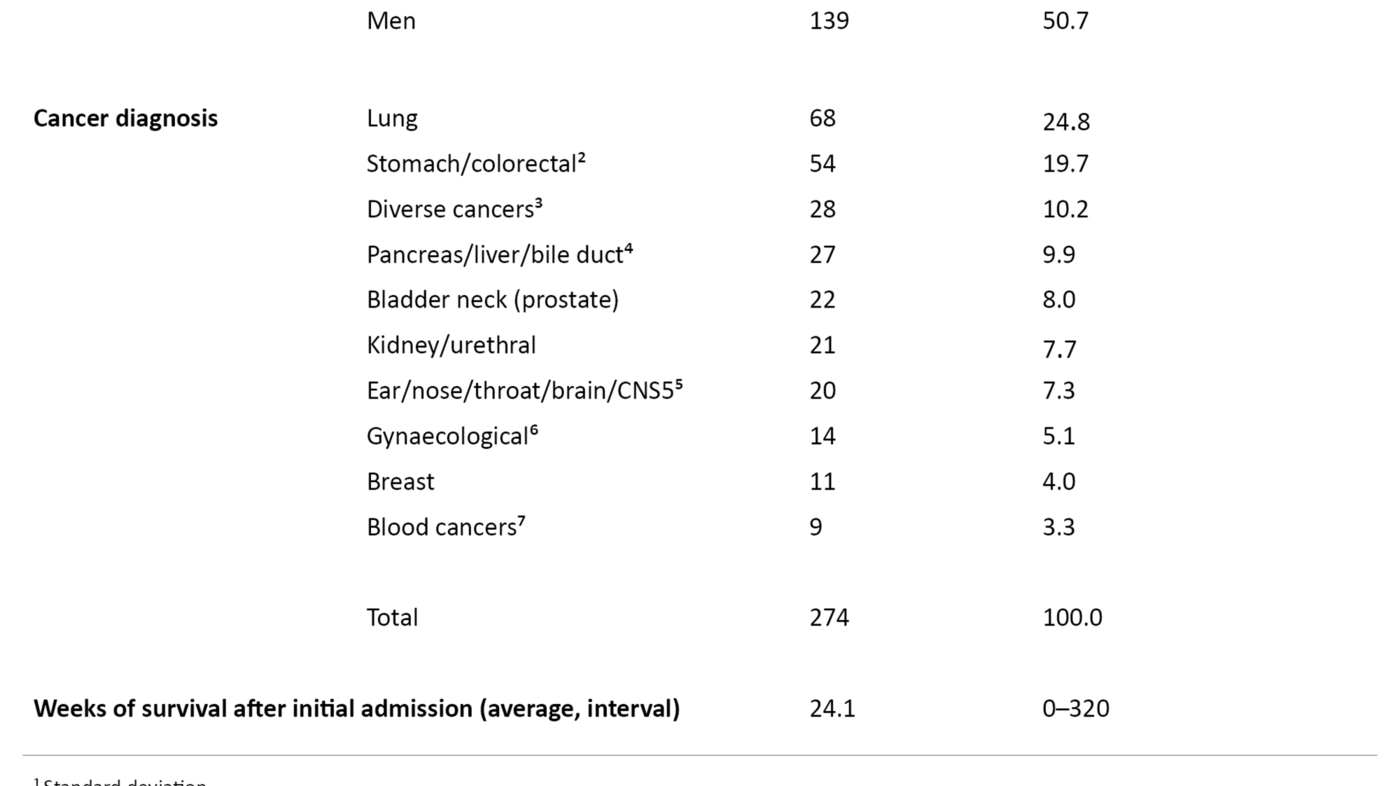
The study presented symptom data from 274 cancer patients, of whom 135 were women and 139 were men. The average age was 69.5 years with a distribution from 38 to 90 years. Average survival after first admission was 24 weeks. Data collection took place in the period from 2008 to 2016. The number of patients who charted the various symptoms is shown in Figures 2, 3 and 4.
Clinical picture on admission
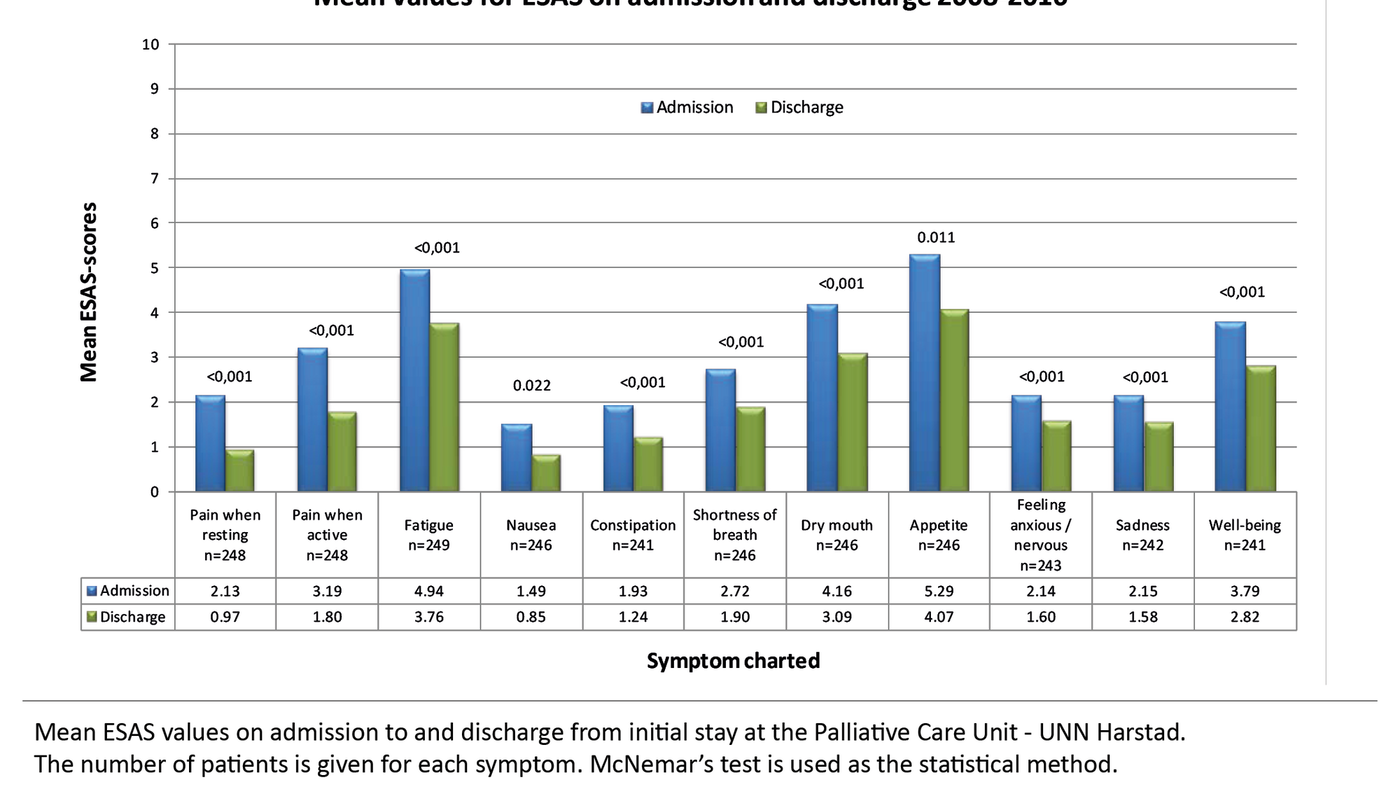
In Figure 2, we present the symptoms dichotomised into the following categories: ‘None’ (score 0), ‘Mild’ (score 1–3), ‘Moderate’ (score 4–6) and ‘Pronounced’ (score 7–10). Fatigue, dry mouth, loss of appetite and sense of well-being (‘Overall, how do you feel today?’) are symptoms which more than 50 per cent of the patients rated as between 4 and 10. We find the largest number of ‘pronounced symptoms’ for loss of appetite (score 7–10).

Clinical picture for women and men
Figure 3 presents the mean symptom burden stratified for men and women. We have omitted patients with breast cancer, gynaecological cancers and prostate cancer.
With the exception of shortness of breath, women report higher values than men for the symptoms charted. However, the differences are only statistically significant for the symptoms fatigue (p = 0.009) and dry mouth (p = 0.005).

Status on admission and discharge
The average length of stay at the unit was 3.3 days (SD 0.93), with 4 days constituting a whole week. Figure 4 shows a reduction in all symptoms after a stay at the palliative care unit. McNemar’s test shows that the change is statistically significant for all the symptoms screened.
Discussion
The health and care services are being evaluated and assessed in respect of waiting time, use of resources and time taken to receive the discharge report. There are few quality indicators that provide information about the quality of palliative care and follow-up received by our most seriously ill patients in palliative care (1). Recommendations were made as early as 2004 on identifying the proportion of patients screened by ESAS as well as the proportion who achieved satisfactory symptom relief (2).
The 2018 Official Norwegian Report on palliation also highlighted ESAS as an effective screening tool (1). National studies show that a systematic approach to the patient using ESAS to provide targeted charting of symptoms provides better information about patients’ problems and enhances nursing quality (16). Rhondali et al. have shown that there is little correlation between the patient’s symptoms and nurses’ impressions of these symptoms (4).
Symptoms
Research that identifies correlations between various symptoms shows that pain is often associated with fatigue, nausea, tiredness and loss of appetite (11). The findings in our study are of interest because the secondary effects of pain seem to be more pronounced than the pain itself. In contrast to pain, these symptoms can be difficult to pinpoint, treat and evaluate without repeated and systematic use of a validated screening tool.
Charting symptoms is of central importance in a palliative disease course in order to give the patient optimal and effective relief for the troublesome symptoms. The aim of charting symptoms, follow-up treatment and evaluation is to achieve the best possible quality of life. Symptoms that patients describe as moderate to pronounced (4–10 on ESAS) should be prioritised in patient treatment.
Loss of appetite
Loss of appetite and cachexia constitute a common problem for palliative patients (22). In our study, 72 per cent of patients have a symptom score of >3 on this question on admission (Figure 2). There may be many causes such as oral fungal infections, changes in sense of taste, difficulties swallowing, obstructions in the GI tract, side effects of radiation therapy or cytostatic treatment, accumulation of fluid in the abdominal cavity (ascites), pain etc. (22).
Cachexia is a negative prognostic factor that is often associated with a shorter life expectancy (22). Loss of appetite alone or together with other symptoms increases the nutritional risk for the patients. In everyday clinical practice, it may be difficult for nurses to identify whether a patient is facing a nutritional risk before significant weight loss is observable (22).
National recommendations stipulate that inpatients in the specialist health service must be nutritionally screened on admission using a validated tool, and should then be monitored on a weekly basis. Measures must be implemented, documented, evaluated and reported to the next treatment level (23).
Poor nutrition increases the symptom burden and reduces quality of life. Early intervention in the form of dietary recommendations and nutritional supplements is therefore crucial, particularly for this patient group (22).
Fatigue
In our study, 69 per cent of patients rate fatigue at an intensity of >3 on admission (Figure 2). Cancer-related fatigue is estimated to affect 90 per cent of the patients (21). The intensity increases during the cancer trajectory (21).
Fatigue is a considerable challenge in cancer care and is often overlooked and therefore inadequately treated (21). The causes of fatigue are complex, and nutrition, pain, side effects of tumour-related treatment and anxiety are important contributory factors. It is therefore vital that nurses have knowledge of the phenomenon.
Dry mouth
A dry mouth (xerostomia) is indicated as a problem for over half of the patients with advanced cancer (24). In our study, 53 per cent rate dry mouth at an intensity of >3 on ESAS. A dry mouth is often due to a combination of different medications, chemotherapy and radiotherapy, and results more readily in infections in the oral cavity. In addition, seriously ill patients experience a decline of the immune system, making them more vulnerable to oral infections (25).
It is vital that nurses pay attention to oral health. Focus on this issue and early implementation of recommended measures may prevent and relieve symptoms. There is a range of measures that can ease a dry mouth, such as good oral hygiene, the use of lozenges or a spray to stimulate saliva secretion, and avoiding sweet foods in addition to frequently drinking copious amounts of water. Medication can also relieve a dry mouth (24, 25).
Constipation
Constipation is reported as a considerable problem for palliative patients (10, 15–17). Nevertheless, only 26 per cent of patients in this study score this at >3. Other studies have revealed that constipation occurs in 50–60 per cent of patients with advanced cancer, and in up to 90 per cent of patients who use opioids (13).
Constipation is often accompanied by a distended abdomen, pain, loss of appetite, nausea, vomiting, headache, restlessness and obstipation diarrhoea (13), and can thus have a considerable negative effect on quality of life. Several publications have emphasised that constipation should be included in ESAS (10, 15, 16), which may have been a contributing factor in its inclusion in ESAS-R.
The low prevalence of constipation problems in the study can partly be explained by good prevention measures at the referral wards. Early referral to the palliative care unit during the disease course may also be a contributory cause.
Well-being
In answer to the question ‘Overall, how do you feel today?’ 53 per cent of the patients give a score of >3 on ESAS. Successful palliative care demands the capacity to interpret the patient’s clinical picture as well as the ability and willingness to alter palliative measures as the disease changes (24).
Clinical experience of using ESAS has shown that the question of well-being can be difficult to answer because the question is seen as fairly vague. Many patients answer this question by saying ‘not too good and not too bad’. Bergh et al. found the same in a 2010 study (26).
The degree of accuracy in answers to this question depends on the nurse taking the time to have a conversation about how the patient feels. It is in conversations about everyday topics that important issues can emerge.
Such a conversation is exemplified by a nurse’s dialogue with a patient with advanced cancer who was in the middle of a house renovation. During the conversation, the patient produced a colour chart and asked the nurse: ‘Do you think I would be happy with this colour in my kitchen?’
The question related of course to the choice of colour but it was equally a desire for confirmation that the patient would live to see the newly painted kitchen. Use of ESAS is often a starting point for good conversations about existential questions, questions that go far beyond the charting of symptoms.
Gender differences
The study shows that women, with the exception of those experiencing shortness of breath, present a greater symptom burden on average than men. The differences are statistically significant in the case of fatigue and dry mouth. In a 2011 study, Culleton et al. found no significant differences in the clinical picture for men and women when gender-specific diagnosis groups were excluded (27).
Earlier research has shown that women experience more opioid-based side effects than men (28). Nevertheless, it is uncertain if the differences in our dataset can be explained by gender-based differences in the metabolisation of opioids. Several studies have discussed fatigue or tiredness in cancer patients in relation to gender-based differences (12, 27, 29).
Our study does not provide an explanation of gender differences in respect of cancer symptoms. Nevertheless, it highlights the importance of focussing on and maintaining an awareness of how patients may experience different symptoms depending on gender.
The effect of a stay at the Palliative Care Unit - UNN Harstad
The study shows a significant decline in all symptoms in both men and women on discharge from our palliative care unit. This is the case regardless of the short duration of hospitalisation and despite the fact that the unit is a five-day ward with an average hospitalisation period of 3.34 days (a whole week = 4 days).
The factors that may explain the improvement in symptoms are that the patients come for planned hospitalisation and assessment. Treatment measures have often been decided prior to admission and these can be implemented immediately after charting the symptoms upon admission. Systematic, repeated measurements of symptoms are crucial to safeguarding accurate and targeted treatment measures.
The use of a validated assessment tool such as ESAS gives nurses and doctors precise measurements of the patient’s symptom burden. Repeated measurements using ESAS are effective because the patients themselves become the key to healthcare personnel accurately evaluating symptoms.
The study’s limitations and sources of error
The study presents the symptom burden of palliative cancer patients at UNN Harstad on their initial admission to the palliative care unit. Our study only monitored patients for a few days and contains no information about the impact of symptom relief on prognosis, life expectancy and quality of life. In order to answer the research questions, we used only ESAS as a screening tool, which naturally constituted a limitation.
Although spiritual and existential needs in addition to the perspectives of the patient’s family are a natural part of holistic, palliative care, our study does not discuss these. The patients in the study included both men and women with different cancer diagnoses and of different ages. Any generalisation of the findings must therefore be made with caution since the patient data are limited.
Many patients said it was difficult to rate a symptom. Some symptoms, such as ‘dry mouth’ and ‘Overall, how do you feel today?’ were described as particularly difficult to define on a numerical scale.
Norwegian research from 2011 revealed a risk that patients may misunderstand ESAS and wrongly interpret the questions. It is therefore necessary to go over the form together with the patient (26), as we did systematically in our study. Eight to ten different nurses, all of whom had received prior training in the use of ESAS, collected the data.
The fact that different nurses helped patients to complete ESAS may have led to variations in the values submitted. The most seriously ill patients were not included.
It is easy to give an inverse response for the ‘appetite’ symptom (good appetite should be ‘0’, no appetite ‘10’). A number of publications have pointed out this phenomenon (26, 30). The personnel at the unit were aware of this potential source of error and avoided erroneous registration.
Conclusion
Proficient nursing entails attention to and knowledge of patients’ symptoms. The study shows that systematic registration of symptom data together with swift implementation of palliative measures to relieve symptoms leads to a diminished symptom burden. Systematic use of ESAS increases the likelihood of discovering symptoms that might otherwise be overlooked.
Loss of appetite, dry mouth and fatigue are the dominant challenges for cancer patients on initial admission to the Palliative Care Unit - UNN Harstad. In a palliative disease course, systematic charting of symptoms, swift start-up of interdisciplinary treatment measures and evaluation of these measures are prerequisites for effective treatment.
The study shows that systematic use of ESAS with rapid implementation of treatment measures gives a reduction in all symptoms charted despite short hospitalisation. Finally, we would emphasise that ESAS cannot replace a proper conversation but is a useful tool for more targeted treatment of symptoms.
Recommendations for further research
One of the main goals of a stay at a palliative care unit is to relieve the patient’s symptoms. This study presents a picture of how palliative patients report their symptoms. The study can be used in future qualitative and quantitative research to underpin how cancer patients in the palliative stage rank their symptoms.
The study also confirms the need for treatment programmes offering expertise in palliation in Norway. It is important to direct focus to cancer patients’ symptoms. Cancer symptoms are complex and challenging for patients, their families, and health personnel.
The results of this study can have clinical implications for the nursing of palliative patients. For example, further research could focus on the impact of specific nursing measures in respect of symptom relief and improved quality of life.
The study has not received external funding, and the authors report no conflicts of interest.
We wish to thank all the nurses at the Palliative Care Unit - UNN Harstad, who have contributed with great enthusiasm and dedication to the charting of symptoms in ESAS. Our thanks also go to senior consultant and former head of department Per Christian Valle, who has been a key driver in the establishment and development of the unit. Last but not least, we wish to thank our patients.
References
1. NOU 2017: 16. På liv og død. Palliasjon til alvorlig syke og døende. Oslo: Departementenes sikkerhets- og serviceorganiasasjon, Informasjonsforvaltning; 2017. Available at: https://www.regjeringen.no/contentassets/ed91baf5d25945b1a0b096c0ce376930/no/pdfs/nou201720170016000dddpdfs.pdf(downloaded 07.12.2018).
2. Engstrand P, Haugen DF, Hessling SE, Jordhøy M, Kaasa S, Kristiansen B, et al. Standard for palliasjon: Norsk forening for palliativ medisin; 2004. Available at: https://legeforeningen.no/Emner/Andre-emner/Publikasjoner/Standard/Standard-for-palliasjon/(downloaded 20.12.2018).
3. Loge JH, Haugen DF, Aass N, Huurnink A, Skarholt K, Nandrup E, et al. Nasjonalt handlingsprogram med retningslinjer for palliasjon i kreftomsorgen. Oslo: Helsedirektoratet; 2007. Available at: http://helsedirektoratet.no/publikasjoner/nasjonalt-handlingsprogram-med-retningslinjer-for-palliasjon-i-kreftomsorgen-/Sider/default.aspx(downloaded 20.12.2018).
4. Rhondali W, Hui D, Kim SH, Kilgore K, Kang JH, Nguyen L, et al. Association between patient-reported symptoms and nurses' clinical impressions in cancer patients admitted to an acute palliative care unit. J Palliat Med. 2012;15(3):301–7.
5. Stromgren AS, Groenvold M, Sorensen A, Andersen L. Symptom recognition in advanced cancer. A comparison of nursing records against patient self-rating. Acta Anaesthesiol Scand. 2001;45(9):1080–5.
6. Seow H, Sussman J, Martelli-Reid L, Pond G, Bainbridge D. Do high symptom scores trigger clinical actions? An audit after implementing electronic symptom screening. J Oncol Pract. 2012;8(6):e142–e8.
7. Guner CK, Akin S, Durna Z. Comparison of the symptoms reported by post-operative patients with cancer and nurses’ perception of patient symptoms. European Journal of Cancer Care. 2014;23(4):523–30.
8. Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7(2):6–9.
9. Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer. 2000;88(9):2164–71.
10. Myhra CB, Grov EK. Sykepleieres bruk av Edmonton Symptom Assessment Scale (ESAS). Sykepleien Forskning. 2010;5(3):210–8. DOI: 10.4220/sykepleienf.2010.0113.
11. Cheung WY, Le LW, Gagliese L, Zimmermann C. Age and gender differences in symptom intensity and symptom clusters among patients with metastatic cancer. Support Care Cancer. 2011;19(3):417–23.
12. Miaskowski C. Gender differences in pain, fatigue, and depression in patients with cancer. J Natl Cancer Inst Monogr. 2004(32):139–43.
13. Helsedirektoratet. Nasjonalt handlingsprogram for palliasjon i kreftomsorgen. Oslo: Helsedirektoratet; 2015.
14. Watanabe SM, Nekolaichuk CL, Beaumont C. The Edmonton Symptom Assessment System, a proposed tool for distress screening in cancer patients: development and refinement. Psychooncology. 2012;21(9):977–85.
15. Strand E, Ellingsen E. Hvordan har du det i dag? Sykepleien. 2007;95(17):60–64. 2009. DOI: 10.4220/sykepleiens.2007.0050.
16. Slåtten K, Fagerström L, Hatlevik OE. Clinical competence in palliative nursing in Norway: the importance of good care routines. Int J Palliat Nurs. 2010;16(2):80–6.
17. Watanabe S, Nekolaichuk C, Beaumont C, Mawani A. The Edmonton Symptom Assessment System –what do patients think? Support Care Cancer. 2009;17(6):675–83.
18. Watanabe SM, Nekolaichuk C, Beaumont C, Johnson L, Myers J, Strasser F. A multicenter study comparing two numerical versions of the Edmonton Symptom Assessment System in palliative care patients. J Pain Symptom Manage. 2011;41(2):456–68.
19. Helsedirektoratet. ICD-10. Den internasjonale statistiske klassifikasjonen av sykdommer og beslektede helseproblemer [Internet]. Oslo: Helsedirektoratet [updated 01.01.2018, cited 20.12.2018]. Available at: https://finnkode.helsedirektoratet.no.
20. Selby D, Cascella A, Gardiner K, Do R, Moravan V, Myers J, et al. A single set of numerical cutpoints to define moderate and severe symptoms for the Edmonton Symptom Assessment System. J Pain Symptom Manage. 2010;39(2):241–9.
21. Lynch MT. Palliative care at the end of life. Semin Oncol Nurs. 2014;30(4):268–79.
22. Nordøy T, Thorsen L, Kvikstad A, Svendsen R. Ernæring og væskebehandling til pasienter med ikke-kurabel kreftsykdom. Tidsskrift for Den norske legeforening. 2006;126(5):624–7.
23. Guttormsen AB, Hensrud A, Irtun Ø, Mowé M, Sørbye LW, Thoresen L, et al. Nasjonale faglige retningslinjer for forebygging og behandling av underernæring. Oslo: Helsedirektoratet; 2009.
24. Nordøy T, Svendsen R, Johansen MJ, Buitink M, Ervik B, Aasebøe U, et al. Håndbok i lindrende behandling: Universitetssykehuset i Nord-Norge – Lindring i Nord; 2012.
25. Trier EL, Jørstad C. Munnstell av alvorlig syke. Sykepleien. 2014;102(9):58–61. DOI: 10.4220/sykepleiens.2014.0108.
26. Bergh I, Kvalem IL, Aass N, Hjermstad MJ. What does the answer mean? A qualitative study of how palliative cancer patients interpret and respond to the Edmonton Symptom Assessment System. Palliat Med. 2011;25(7):716–24.
27. Culleton S, Dennis K, Koo K, Zhang L, Zeng L, Nguyen J, et al. Gender difference in symptom presentations among patients with bone metastases in gender-specific and gender-neutral primary cancers. World J Oncol. 2011;2(3):102–12.
28. Fillingim RB, Ness TJ, Glover TL, Campbell CM, Hastie BA, Price DD, et al. Morphine responses and experimental pain: sex differences in side effects and cardiovascular responses but not analgesia. J Pain. 2005;6(2):116–24.
29. Sigurdardottir KR, Haugen DF. Prevalence of distressing symptoms in hospitalised patients on medical wards: A cross-sectional study. BMC Palliat Care. 2008;7:16.
30. Watanabe SM, Nekolaichuk CL, Beaumont C. Palliative care providers' opinions of the Edmonton Symptom Assessment System Revised (ESAS-r) in clinical practice. J Pain Symptom Manage. 2012;44(5):e2–e3.










Comments