COVID-19 and the management and care of peripheral intravenous catheters
Summary
Background: During the COVID-19 pandemic, hospitals implemented several infection control measures that could affect the quality of insertion and management of peripheral intravenous catheters (PIVCs). Pre-pandemic studies found a high rate of PIVC complications, but no studies to date have assessed the quality during the pandemic period.
Objective: This prospective study aimed to investigate the effect of the pandemic on PIVC management and care.
Method: Data were collected from adult patients (>18 years) at a university hospital in Norway during a three-week period in February 2020 (pre-pandemic: PP group) and a three-week period in October 2020 (pandemic: P group). The primary outcome measure was PIVC quality as determined by the validated PIVC Mini Questionnaire (PIVC-miniQ), which consists of 16 items relating to deviations from best practice.
Results: The study included 483 PIVCs and 413 patients. Of these, 238 PIVCs (49.3%) were collected in the PP group and 245 (50.7%) in the P group. In the PP group, 41.8% of the PIVCs were 18 gauge (green PIVC) or larger, compared to 53.3% in the P group. The median PIVC-miniQ score was 2 in both groups, with a range of 0–10 in the PP group and 0–8 in the P group. Improved quality in terms of fewer patients reporting pain was registered in the P group (14.3% compared to 6.7%), and 36.3% had blood in the IV line in the PP group, compared to 26.5% in the P group. However, a poorer quality was observed in the P group for the PIVC-miniQ items ‘purulence’ (0% in the PP group compared to 6% in the P group) and ‘loose dressing’ (15.2% in the PP group compared to 24.9% in the P group). Documentation of PIVC insertion and indication was equally insufficient in both groups (p = 0.857).
Conclusion: Although some differences were observed at item level, the PIVC quality as measured by the PIVC-miniQ total score remained unchanged during the pandemic. Given the low workload of our hospital during the pandemic, it is a paradox that the PIVC care did not improve.
Cite the article
Helland E, Høvik L, Gustad L, Gjeilo K. COVID-19 and the management and care of peripheral intravenous catheters. Sykepleien Forskning. 2024;19(95645):e-95645. DOI: 10.4220/Sykepleienf.2024.95645en
Introduction
Insertion of peripheral intravenous catheters (PIVCs) is one of the most frequently executed invasive procedures in hospital patients and is associated with a risk of complications such as insertion difficulties, dislodgement, infiltration, occlusion, phlebitis and bloodstream infections (1-6).
Clinical signs at the insertion site often used in phlebitis assessment are pain, erythema, oedema, warmth, purulence, streak formation, hard tissue and palpable vein (7). A bloodstream infection due to a PIVC is considered the most serious of all hospital-acquired infections and was listed as one of the top ten risks for patient safety in 2019 (8, 9).
Hospital-acquired infections may lead to a prolonged hospital stay, increased morbidity and mortality, increased use of antibiotics as well as increased public health costs (1, 8, 9). A systematic review from 2017 found that 22% of intravenous catheter-related bloodstream infections were associated with PIVCs (4), and in Spain, PIVC-related bloodstream infections have been shown to increase in general wards (10).
In Norway, primary bloodstream infections account for 7% of all hospital-acquired infections (11). One study from Norway found that about 8% of all bloodstream infections caused by S. Aureus stemmed from an IV catheter, and 25% stemmed from an unknown source (12), which may have been a PIVC.
The PIVC size, insertion site, number of PIVCs inserted, dressing securement, lack of documentation and PIVC dwell time are associated with a risk of complications (13). International guidelines recommend avoiding insertion of PIVCs in anatomical areas with flexion, as well as choosing a size of PIVC suited for the intended use (14). PIVCs should be stabilised with a sterile, clean, dry and intact dressing (13). The date of insertion should be documented both on the dressing and in the patient journal (1).
PIVCs in use should be replaced on clinical indication only (15, 16) and idle PIVCs should be removed promptly (14). PIVC complications can be prevented by following evidence-based steps to ensure best practice in the insertion and management of PIVCs. This includes daily evaluation of the PIVC and insertion site, with bedside assessment of complications and adherence to guidelines for infection prevention (13, 16-18).
The COVID-19 pandemic brought about a natural experiment, with new demands that could have interfered with PIVC quality: for example, demands on infection control, use of personal protection equipment, new recommendations for distance without protection equipment, decreased possibility to separate clean and unclean zones and mental and ethical strains that could affect overall work quality (19-21).
The main aim of this study was to assess adherence to evidence-based recommendations on PIVC management and care using the validated PIVC Mini Questionnaire (PIVC-miniQ) assessment tool (22) at a Norwegian university hospital during the first pandemic wave compared to a pre-pandemic survey.
Method
Design
The quality of PIVC care was measured prospectively using the PIVC-miniQ assessment tool (22) in two cross-sectional unannounced prevalence surveys. The first of these was conducted in February/March 2020 (pre-pandemic group: PP group) and the second in October/November 2020 (pandemic: P group).
The study solely included PIVCs, and quality of care for midline catheters or peripherally inserted central catheter lines was therefore not measured. The surveys were conducted in weeks 9, 10 and 11 in 2020 for the PP group and weeks 43, 44 and 45 for the P group.
Participants and setting
The study was conducted at a Norwegian university hospital with approximately 1000 inpatient beds. This is a local hospital for 327 000 people and serves as a reference hospital for 730 000 inhabitants.
Each prevalence survey recruited a convenience sample of adult patients (>18 years) admitted to a medical or surgical inpatient ward or intensive care unit (ICU), covering all 14 wards and units. All patients with PIVCs that were available at their unit at the time of screening were included, except patients who were unable to receive information about the study or give verbal consent.
Screening during the COVID-19 pandemic was conducted according to a standard operations procedure (SOP) developed to mitigate the risk of COVID-19 transmission during data collection. Patients diagnosed with COVID-19 were not included in the study due to restrictions on infection control equipment.
Data collection
The project leader and nurses from the Department of Infection Control at the hospital oversaw the project and collected data using the PIVC-miniQ together with nurse educators. The screenings were conducted on weekdays after 11 a.m. to ensure that patients were not occupied with breakfast or doctor rounds. To avoid increasing the focus on PIVC management, the exact time of data collection was not announced in advance to the staff at the screening sites.
Observation of each PIVC while completing the PIVC-miniQ generally took 3-4 minutes, and the patient records were checked for indication and documentation of PIVC immediately after the observation. Due to infection control measures during the pandemic period, the departments insisted on their own nurses carrying out the PIVC surveys in the P group. Meanwhile, the PP group was measured by nurses trained to conduct the survey.
Details of the PIVC-miniQ
The PIVC-miniQ evaluates PIVC management and care (22) in line with recommendations in international evidence-based literature and best practice guidelines (18), and it has been validated in two countries (22, 23). An English version of the PIVC-miniQ has previously been published (22). The validated Norwegian version that was used in this study has been published previously, see Additional file Figure SI. In a previous study, the intraclass correlation (ICC) between 64 Norwegian raters was 0.678 at one hospital and 0.577 at a second hospital (22).
The PIVC-miniQ contains two sections (22). The first section includes general information about patient age and gender, PIVC dwell time, PIVC size, insertion site and context of insertion setting (paramedic, emergency department, operating room, inpatient ward/ICU or computer tomography (CT)/magnetic resonance (MR) lab).
The second section lists 16 clinical observations. Of these, eight observations are clinical signs of complications at the insertion site (i.e. pain, erythema, oedema, warmth, purulence, streak formation, hard tissue and palpable vein), and these are summed up to form a phlebitis score (continuous score 0-8 phlebitis signs). One sign of phlebitis was enough to categorise phlebitis, and a higher score indicated several phlebitis signs.
The other eight observations are related to dressing/equipment, documentation and indication for PIVC. All of these 16 observations are either present (1 point) or absent (0 points). A total score ranging from 0 to 16 can be calculated for each PIVC, where a higher score indicates increasing deviation from best practice in PIVC management and care.
The PIVC-miniQ was printed in a machine-readable format. One form was completed per PIVC and the forms were scanned at the Clinical Research Unit at the local university.
Research ethics
This study was regarded as a health quality research project and no directly identifiable patient information was collected. The Norwegian Centre for Research Data (NSD), now called Sikt - Norwegian Agency for Shared Services in Education and Research, therefore considered informed verbal consent to be adequate under Norwegian legislation. Participants gave verbal consent to the screening and collection of information from their patient records.
The study was approved by the medical director at the hospital and adhered to guidelines for quality improvement studies. No data protection impact assessment was needed as the risk from processing anonymous personal data is minimal.
Analysis
Descriptive and comparative analyses were performed to address the research question. Categorical data were reported as frequencies (n) and percentages (%), and continuous data as median (range) and/or mean (SD, standard deviation) as appropriate. A univariate analysis of categorical data was performed to describe frequencies and percentages. As women are more likely to report pain with phlebitis (6), we checked the distribution of men and women in both the pre-pandemic and pandemic groups.
The distribution of continuous data was checked using the Kolmogorov-Smirnov test of normality, histograms and a Q-Q plot. Data were found to be skewed, and the Mann-Whitney U test was therefore used to compare continuous sum scores between the first and the second survey. Categorical data were compared using Pearson’s chi-square or Fischer-Freeman-Halton’s exact test as appropriate for cell counts.
We tested the differences in documentation and indication of PIVCs between the PP and P groups in each insertion setting (paramedic, emergency department, ward/ICU, unknown, overall). This was done by combining the proportion (%) of missing date on PIVC, missing documentation in the patient record and indication for use in each insertion setting for the PP and P groups. This approach reduced the number of statistical tests from 18 to six, thus reducing the probability of a false positive finding.
For all comparisons, a 2-sided p value < 0.05 was considered statistically significant. Data analyses were performed using IBM SPSS Statistics version 27 and STATA/SE version 17.0.
Results
We approached 413 patients and all consented to participate in the study. As shown in Table 1, the PIVC-miniQ was used to observe a total of 483 PIVCs in 413 patients. The PP group included 238 (49.3%) PIVCs in 199 (48.2%) patients and the P group included 245 (50.7%) PIVCs in 214 (51.8%) patients. Age (p = 0.791) and number of catheter days (p = 0.244) were similar in the two groups. The percentage of men (59.8%) compared to women (40.2%) was higher in the PP group than in the P group (p = 0.002).
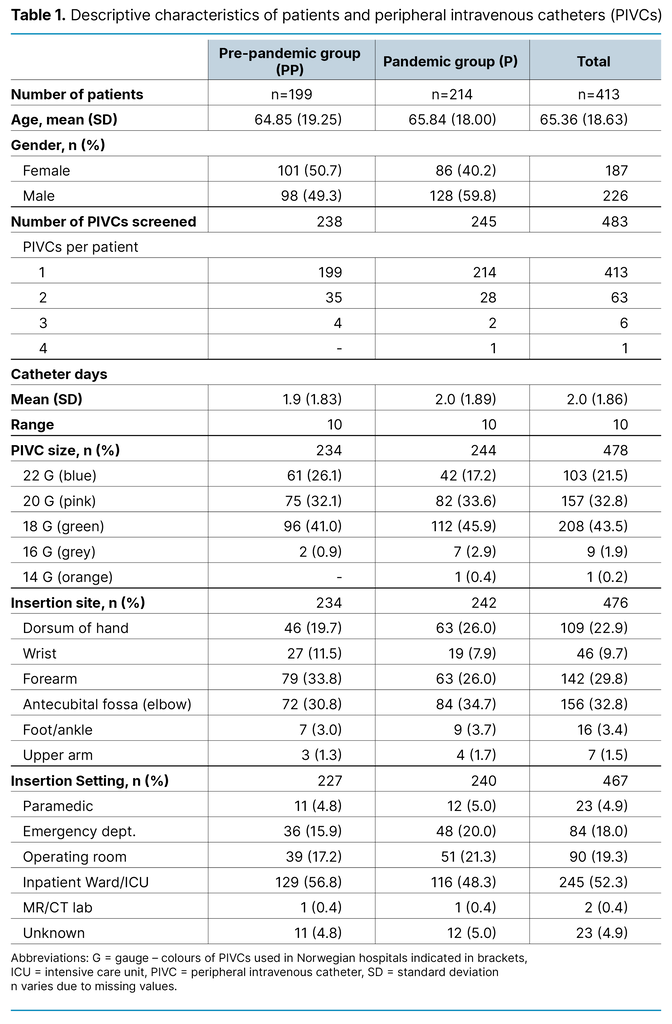
Table 1 shows descriptive data for demographic variables and the first section of the PIVC-miniQ. In the PP group, 41.9% of the PIVCs were 18 gauge (green PIVC) or larger, compared to 49.2% in the P group. Placement in non-recommended sites such as the wrist was 11.5% in the PP group and 7.9% in the P group, and antecubital fossa was 30.8% in the PP group compared to 34.7% in the P group.
Figure 1 shows adherence to the evidence-based practice recommendations measured by the PIVC-miniQ for the PP group and P group. The median score was 2 in both the PP group (range 0-10) and the P group (range 0-8), with no difference between the PIVC-miniQ score between groups (p = 0.414).
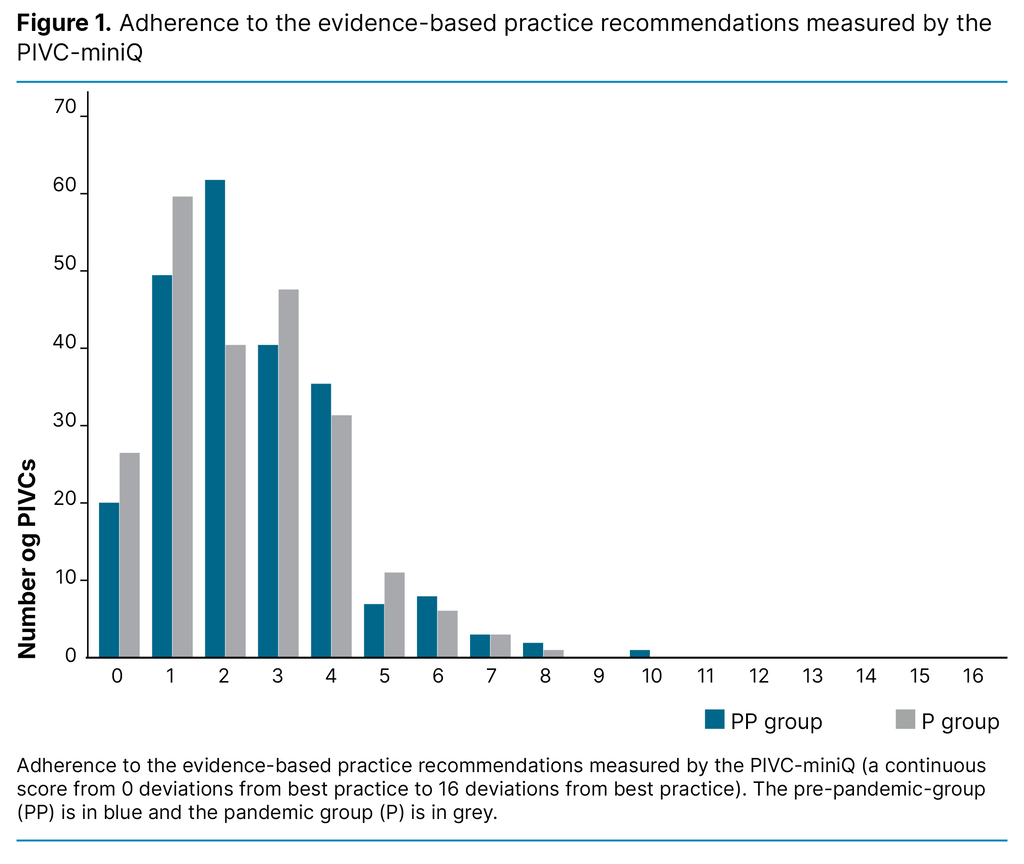
Table 2 shows the results for each item in the PIVC-miniQ. For signs of phlebitis, the most frequent problem was pain and erythema in both the PP group and the P group. There was a significant reduction of reported pain from the PP group to the P group (14.3% compared to 6.7%, p = 0.006). Women reported having pain more frequently in both the PP group (20% of women compared to 9% of men, p = 0.02) and the P group (11% of women compared to 4% of men, p = 0.04).
Improved quality of care was observed for the item ‘blood in IV line’, which was found in 36.3% of observations in the PP group compared to 26.5% in the P group (p = 0.02). However, poorer quality was observed for some of the PIVC-miniQ items in the P group: purulence (0% in the PP group compared to 6% in the P group, p = < 0.001) and loose dressing (15.2% in the PP group compared to 24.9% in the P group, p = 0.008).
In the PP group, 77.5% of PIVCs placed in the antecubital fossa were bloodstained, compared to 78.7% in the P group. Results for bloodstained dressings on PIVCs inserted in the forearm were 27.0% in the PP group and 43.2% in the P group. Of the 15 cases of purulence, few patients experienced other signs of infection; only one experienced pain, three erythema, four oedemas and none warmth.
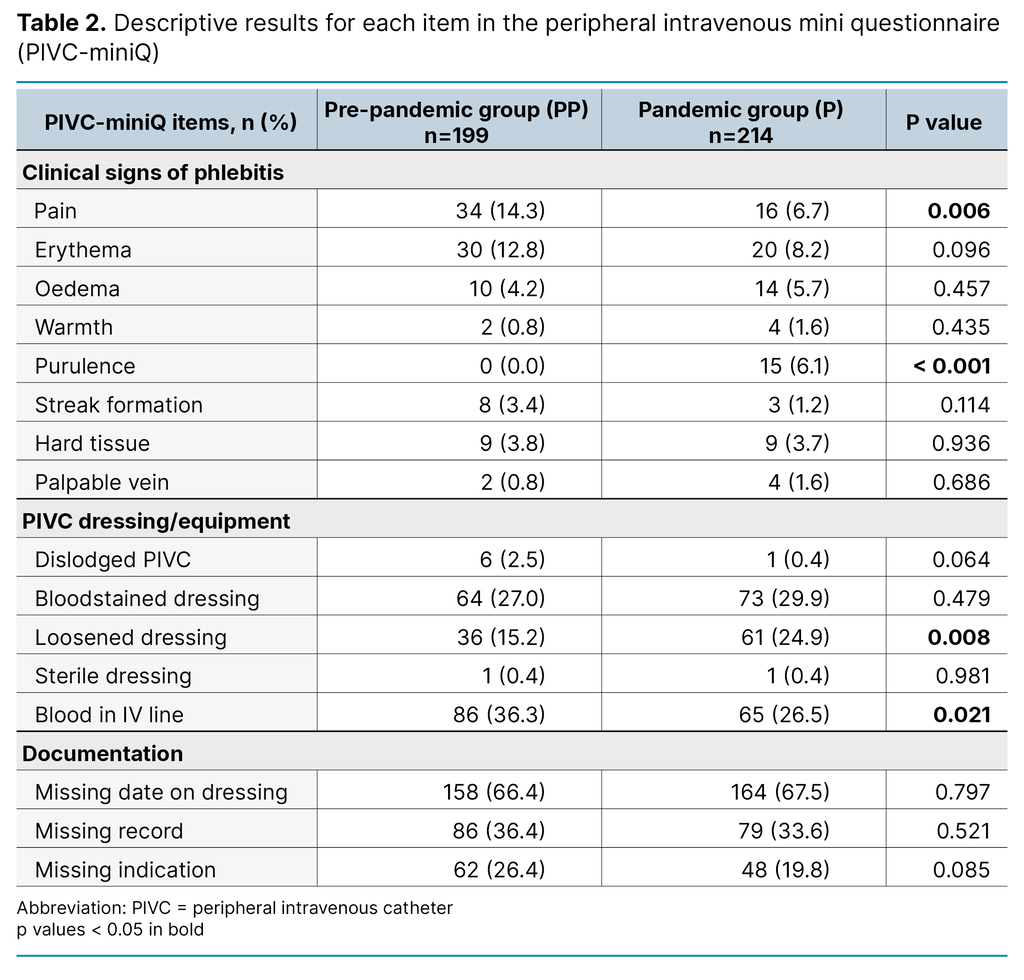
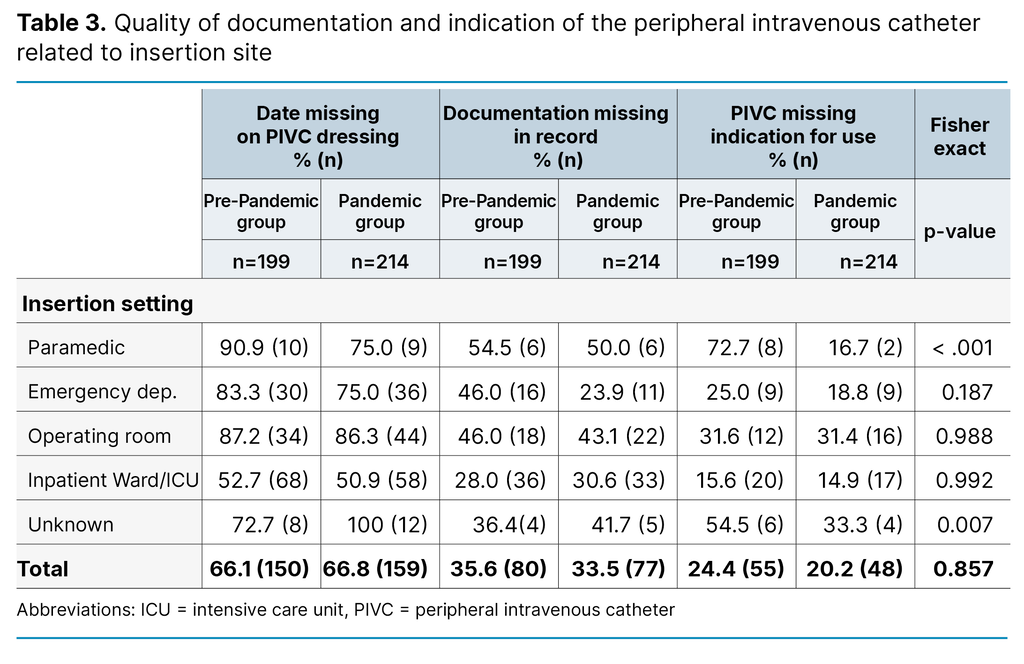
Table 3 shows proportions of documentation of insertion-date on the PIVC dressing or patient journal, and documentation of indication for use in patient records, in the PP group vs the P group. As very few PIVCs were inserted in an imaging lab (see Table 1), the imaging labs was left out of Table 3.
In the pandemic group, a lower proportion of the PIVCs that were inserted in the paramedic setting lacked documentation of insertion-date on dressing, and documentation of PIVC and indication in patient journal, compared to the PP group. Those PIVCs that had unknown insertion site, however, had worsened documentation in the pandemic compared to before. The overall documentation across all departments were equal in the pandemic and pre-pandemic group.
Discussion
We observed some indications of improved quality during the pandemic, such as less pain and fewer PIVCs with blood in the IV line. However, we also observed indications of poorer quality, with more loose bandages and an increased prevalence of purulence.
As the PIVC-miniQ demonstrated higher reliability in its total score, we conclude that overall, this study found that adherence to evidence-based practice recommendations was similar in normal circumstances and during a situation in which pandemic guidelines were enforced. In both surveys, deviations in dressing status, documentation of PIVC indication and insertion were commonplace.
PIVC management and care during the COVID-19 pandemic
Overall, PIVC quality was similar in the PP group and P group, and it thus seems that the COVID-19 pandemic did not substantially affect PIVC management and care. As this is the first study comparing PIVC quality before and during the pandemic, it is not directly comparable to other studies. As PIVC complications include hospital-acquired infections (18), we could make an indirect comparison with a retrospective study from Australia that only found minor variations of hospital-acquired infections between the hospitals of interest before and during the pandemic (24).
Indirect comparisons can also be made with studies that observed an increase in bloodstream infections during the pandemic (25). Other studies have found that hospital-onset bloodstream infections increased substantially during the pandemic period, highlighting the need for infection prevention and control (26, 27).
We did not have access to hospital-onset bloodstream infections in this study, but we found evidence of increased purulence with PIVCs despite an increased focus on hygiene and infection prevention during the pandemic. Purulence is an indication of phlebitis caused by bacteria that may in turn be caused by infection, which is an inflammatory response that entails pain, erythema, oedema and warmth (26).
The proportion of both pain and erythema had decreased from the PP group to the P group, and the number of cases reporting warmth and oedema were few in both groups. The different prevalence of purulence across measurements could be a result of inadequate training in using the PIVC-miniQ during the pandemic as each ward used their own personnel to prevent the transmission of COVID.
As female patients have previously been shown to be more prone to experiencing the phlebitis sign of pain than men (6), it is important to note that, during the pandemic period, fewer women than men were admitted to the wards and units. However, the difference in PIVC-related pain was found in both the PP group and the P group, and the different gender distribution between the groups should not therefore have impacted on the results.
Even though deviations in best practice related to PIVC care were low across measurements, deviations still existed that highlight the importance of ongoing clinical audits and continuous education for healthcare personnel (12). Pain and erythema were the most prevalent deviations, and the prevalence in both surveys was comparable to most other studies. Blanco-Mavillard et al. found that about 5% experienced pain, and erythema was found in 6% of all patients with a PIVC (27).
Alexandrou et al. found that 10% of PIVCs worldwide were painful (1). Høvik et al. previously found 13% pain and 10% erythema, and a similar mean PIVC-miniQ total score of 2.04 (28). One study from Nepal, however, found a much higher prevalence of pain: in approximately > 40% of patients with a PIVC, and a slightly higher mean PIVC total score of 2.37 (23).
The consistent quality observed during the two surveys in this present study could be partly due to the normal or even reduced workload experienced at the time of the pandemic survey (29). Studies from countries that had larger volumes of COVID-19 patients than Norway have found that nurses experienced an increased workload and work stress because of the pandemic (20, 30, 31). It is therefore conceivable that the quality of PIVC care could have been compromised if the patient load had exceeded normal levels.
Strengths and limitations
As this was a single-centre study, the results describe the situation in a university hospital in Norway where the incidence of COVID-19 patients was low in the measurement period. A study period with more COVID-19 cases, and thus more job stress for nurses, could have resulted in lower PIVC quality for the pandemic group. A further limitation is that we did not record how many patients were unavailable (undergoing procedures) or did not consent, nor which medicine was administered via the PIVC.
However, the number of participants in each survey is similar, and there is therefore no reason to believe that one group is less represented than the other. The PIVC screening was unannounced and the study therefore reflects the actual quality of PIVC management and care. A major strength is that all the 14 hospital units were involved in both surveys.
Conclusion
This study found that while some PIVC deviations decreased in prevalence, others increased, and overall, there was little difference in PIVC management and care before and during the first wave of the COVID-19 pandemic. These findings support the idea that nurses are able to maintain consistent PIVC care during challenging situations. However, the pandemic increased the focus on infection control in general.
Nevertheless, it is a paradox that the PIVC care did not improve given the reduced workload in our hospital setting. Thus, our study highlights the challenges of evidence-based PIVC care during a pandemic. Routines for maintaining quality need to be established before the next pandemic. These should include both online training and standards for quality surveillance in pandemic situations.
Conflicts of interest
Lise Tuset Gustad declare a conflict of interest as she is editor-in-chief (EIC) in Sykepleien Forskning. She did not participate in the handling or decision-making process concerning this submission. The manuscript was overseen by an appointed EIC to ensure impartiality and fairness in the review and editorial process.
Open access CC BY 4.0
The Study's Contribution of New Knowledge
Comments